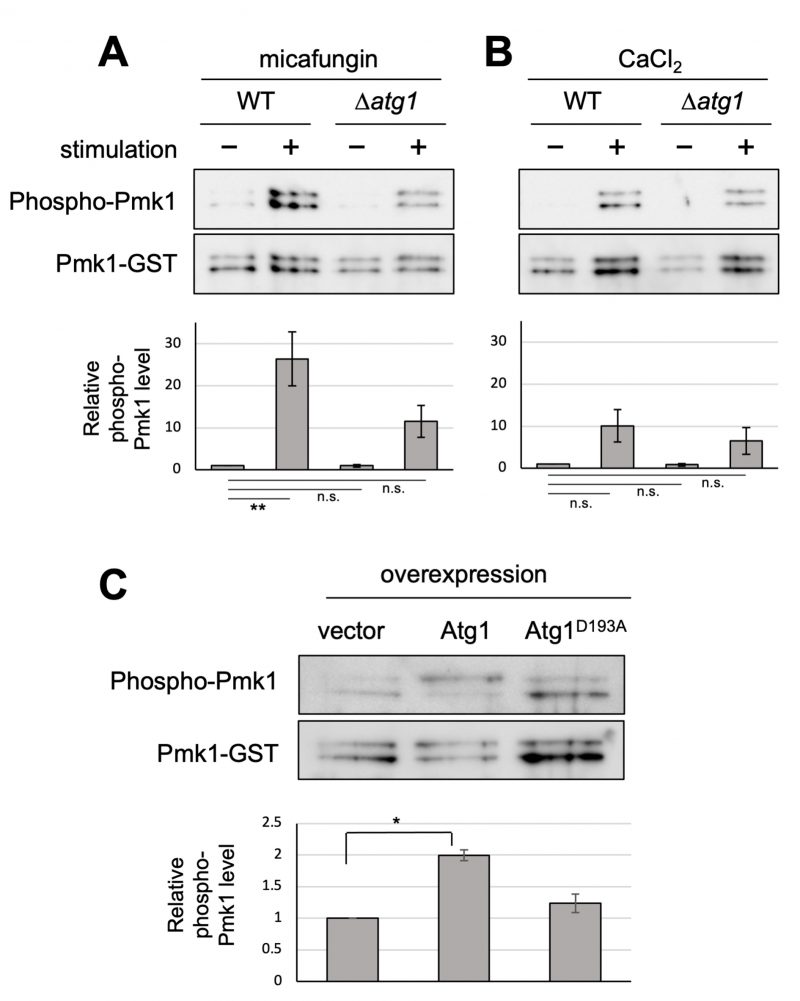
FIGURE 1: Atg1 facilitates the phosphorylation of Pmk1. (A and B) Impact of the deletion of atg1+ on Pmk1 phosphorylation. Wild-type (WT) and Δatg1 cells expressing the C-terminal GST-tagged Pmk1 from the endogenous pmk1 promoter were grown in EMM supplemented with 2 µg/ml micafungin (A) or with 200 mM CaCl2 (B) for 60 min at 27°C. Cell lysates bound to glutathione beads were immunoblotted with anti-phospho-ERK antibodies and anti-GST antibodies to detect phosphorylated Pmk1 and Pmk1-GST (loading control), respectively. Upper panel: representative immunoblot. Lower panel: Relative quantification of phosphorylated Pmk1 normalized by Pmk1-GST. Bar graphs show relative values to WT cells grown in the absence of micafungin nor CaCl2 as the mean ± standard error of the mean (SEM) of five independent experiments. N = 5; **p < 0.01 as assessed by a one-way ANOVA followed by the Dunnett's test for multiple comparisons. n.s. not significant. (C) Impact of overexpression of atg1+ on the Pmk1 phosphorylation. Δatg1 cells expressing Pmk1-GST under the endogenous pmk1 promoter and harboring pREP1-GFP, pREP1-atg1+-GFP, or pREP1-atg1D193A-GFP were grown in EMM without thiamine for 20 hr at 27°C. Cell lysates bound to glutathione beads were immunoblotted with anti-phospho-Pmk1 and anti-GST antibodies and quantified as described in (B). Bar graphs represent the mean ± SEM (N = 3; *p < 0.05 as assessed by Welch's two sample t-test). The levels of overexpression of WT Atg1 and kinase dead Atg1 were approximately equal (Figure S1).
By continuing to use the site, you agree to the use of cookies. more information
The cookie settings on this website are set to "allow cookies" to give you the best browsing experience possible. If you continue to use this website without changing your cookie settings or you click "Accept" below then you are consenting to this. Please refer to our "privacy statement" and our "terms of use" for further information.