Research Articles:
Microbial Cell, Vol. 11, No. 1, pp. 254 - 264; doi: 10.15698/mic2024.07.830
Pathogenic Escherichia coli change the adhesion between neutrophils and endotheliocytes in the experimental bacteremia model
1 Research Laboratory of Scanning Probe Microscopy, Lobachevsky State University of Nizhny Novgorod, Nizhny Novgorod, Russia. 2 Nanotechnology and Biotechnology” Department, Nizhny Novgorod State Technical University named after Alekseev R.E., Nizhny Novgorod, Russia. 3 Nizhny Novgorod Research Institute of Epidemiology and microbiology named after Blokhina I.N., Nizhny Novgorod, Russia.
Keywords: neutrophils, endothelial cells, Escherichia coli, adhesion force, adhesion work, FS spectroscopy, apoptosis, stiffness of membrane-cytoskeleton complex.
Received originally: 13/12/2023 Received in revised form: 13/06/2024
Accepted: 03/07/2024
Published: 22/07/2024
Correspondence:
Svetlana N Pleskova, 603922 Russia, Nizhny Novgorod, Gagarina ave. 23, build. 3, 241; pleskova@mail.ru
Conflict of interest statement: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Please cite this article as: Svetlana N Pleskova, Nikolay A Bezrukov, Sergey Z Bobyk, Ekaterina N Gorshkova, Dmitri V Novikov (2024). Pathogenic Escherichia coli change the adhesion between neutrophils and endotheliocytes in the experimental bacteremia model. Microbial Cell 11: 254-264. doi: 10.15698/mic2024.07.830
Abstract
Septicemia caused by gram-negative bacteria is characterized by high death rate due to the endotoxin release. Since the septicemia depends not only on biochemical aspects of interactions in the system bloodstream, the study of mechanical interactions is also important. Using a model of experimental septicemia caused by E. coli, a hyperproduction of integrins CD11a and CD11b by neutrophils was shown, but this did not lead to the establishment of strong adhesion contacts between endothelial cells and neutrophils. On the contrary, adhesion force and work, as assessed by FS spectroscopy, were statistically significantly reduced in the presence of bacteria. It has also been shown that exposure to the pathogenic strain E. coli 321 increases the stiffness of the membrane-cytoskeleton complex of endothelial cells and bacteria significantly change their morphology on long-term observation. At the same time, we observed the death of neutrophils by apoptosis. Thus, it was shown that besides lipopolysaccharide release there are other pathogenic factors of E. coli: decrease in the interaction between neutrophil and endothelial cell caused by an increase of the endothelial cell rigidity and apoptotic death of neutrophils probably as a result of adhesins and exotoxin effects. Obtained results should be taken in mind during the therapy of septicemia.
INTRODUCTION
Septicemia is characterized by a generalized lightning-like course, severe intoxication, hyperuria and absence of purulent tissue foci. Typically, gram-negative bacteria cause a more severe course of sepsis due to endotoxin release. For example, Mayor-Lynn K. et al. (2005) showed that neonatal sepsis caused by Escherichia coli resulted in a longer stay in intensive care, greater need for mechanical ventilation and higher mortality than sepsis caused by group B Streptococcus 1. Greater mortality was also observed in older patients with out-of-hospital pneumonia complicated by sepsis when the etiological factor was E. coli compared with pneumococcal pneumonia 2. In ventilator-associated pneumonia (VAP) complicated by sepsis, gram-negative microorganisms (Acinetobacter, Pseudomonas, Klebsiella, E. coli) are also predominant among the strains isolated in both pediatric3 and adult4 hospitals. In the pathophysiology of sepsis and bacteremia, the interaction between all the main participants in the events occurring in the bloodstream is important: (1) bacterial strains; (2) neutrophils, which are the urgent response link; (3) endothelial cells, which directly regulate the diameter of the vessels and the degree of permeability to neutrophils during bacterial invasion, thus determining both the rate of development of the pathological process and the compensatory effects on the part of the human body. For example, Amunugama K. et al. (2022)5 showed that a pathogenic strain of E. coli caused greater changes in the lipid profile of endotheliocytes than the non-pathogenic strain, and the addition of neutrophils to the system resulted in an increase in triglycerides in response to the pathogenic strain and an increase in phosphatidylglycerol and diglycerides in response to both strains (pathogenic and non-pathogenic); shifts in the lipid profile of neutrophils under the influence of bacteria were also observed. Thus, in the pathophysiology of septic lesions, it is important to take into account changes in both neutrophils and endothelial cells, since latter play an important role in the regulation of all parts of the pathological process such as: changes in vascular permeability in the process of transendothelial migration, diameter of vascular lumen changes due to NO production, blood coagulation with the risk of life-threatening complications (e.g. DIC), etc. The biochemical aspects of septic process generation have been described in sufficient detail, but the biophysical features of septic invasion development are more difficult to assess due to the limited number of methods that allow the determination of changes in the elastic-mechanical properties of cells in relation to their morpho-functional properties.
The development of new approaches to high-resolution imaging, combined with additional capabilities, has been made possible by various methodological branches of scanning probe microscopy (SPM) as the first microscopic field that not only allows dynamic observations of cells at high resolution, but also intervenes in physiological processes in real time, clearly monitoring the feedback 6. In particular, atomic force microscopy (AFM) has developed a method to record the force and work of adhesion between a neutrophil granulocyte fixed to the surface of an AFM cantilever and an endothelial cell grown on a substrate, a Petri dish 7.
In the study presented below, scanning ion-conductance microscopy (SICM) was used to evaluate endothelial cell stiffness in the E. coli-induced experimental septicemia model and AFM was used to determine for the first time (1) the E. coli effect on the force and work of adhesion in the endothelial cell-neutrophil-granulocyte system and (2) the change in endothelial cell stiffness under the influence of E. coli.
RESULTS
E. coli 321 strain was chosen to study effect of E. coli on the interaction between neutrophils and endotheliocytes. It was isolated from blood of a patient with septicemia. As a result of genotyping, the following pathogenicity factors of the strain were identified (in brackets – abbreviated gene name): possibilities biofilm production (agn43, upaH); Nutritional/Metabolic factor (entF, irp1, fepA, chuA, iroC, iroN, sitC, sitA, sitD, sitB); motility (cheA, flhA, fliF, fliI, fliG, fliM, cheB): invasion (kpsC, kpsD, kpsE, kpsS, kpsF, aslA, ibeC, tia); effector delivery system (gspD, espL1, tssH, espC, tsh, pic, vgrG); adherence (eaeH, upaG/ehaG, fimD, hcpB, papC, sfaX, papX, papB, papI, fimD, fimF, fimG, papG, ydeQ, fimA, ydeR, papE, papF); exotoxin (usp, hlyE/clyA, hlyA, cnf1, cta, cfa, cka, cdtB, cdtA, cdtC).
Thus, strain E. coli 321 mainly carries pathogenicity factors similar in nucleotide sequence with genes of strains related to the category UPEC (uropathogen E. coli). However, it contains ksp genes, which are responsible for formation of K-capsules (K1 capsule – Invasion). These genes are characteristic only for the NMEC (neonatal meningitis-causing E. coli) category. Additionally, virulence genes are often similar to genes of strains containing cnf1 (cytotoxic necrotizing factor 1), which is also a characteristic of the NMEC category. So, most likely strain E. coli 321 is a hybrid that belongs to NMEC. The main characteristics of the NMEC category are: strains possessing the K1 capsular polysaccharide are predominant (approximately 80%) among isolates from neonatal E. coli meningitis and most of these K1 isolates are associated with a limited number of O types (e.g., O-18, O-7, O-1). A natural route of infection (e.g., oral), gut colonization and translocation, dissemination to deeper tissues, and a level of bacteremia necessary prior to the penetration of the blood-brain barrier. E. coli K1 invades brain microvascular endothelial cells (BMECs) via a zipper mechanism and transmigrates through BMECs in an enclosed vacuole without intracellular multiplication 8.
Also, two plasmids were found : IncFIB (E. coli K-12 plasmid F DNA) and IncFII (E. coli plasmid pC15-1a); the first one encodes the F-sex factor, and on the second one resistance to beta-lactam antibiotics, streptomycin/spectinomycin and sulphonamides is encoded.
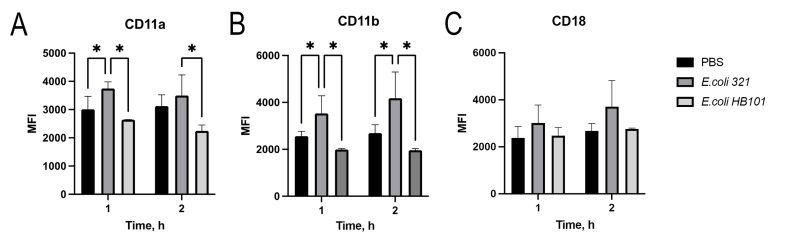
To determine how the surface expression of the major neutrophil integrins (CD11a/CD18 and CD11b/CD18), which play a key role in the migration of polymorphonuclear leukocytes 9, changes under the influence of E. coli, we examined their expression levels after 1 and 2 h of incubation (Figure 1). Two strains of E. coli were studied – pathogenic strain E. coli 321 and commensal strain E. coli HB101. Obviously, after 1 hour of co-incubation of neutrophils with E. coli 321, there was a statistically significant increase in the surface expression of CD11a and CD11b, while the expression of CD18 did not change. The saprophytic strain E. coli HB 101 did not cause any change in the expression of neutrophil receptors during two hours after the start of incubation, and the expression levels of CD11a, CD11b as well as CD18 were the same as the control level.
–
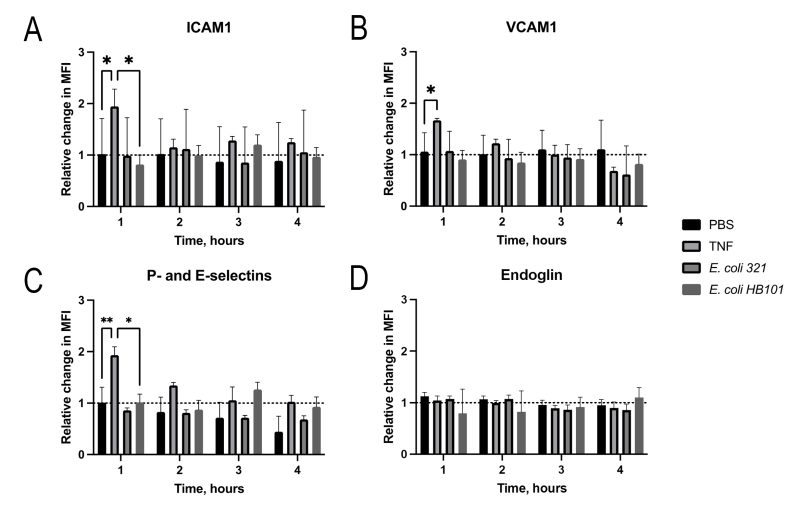
The dynamics of the surface expression of adhesins (ICAM1 and VCAM1), selectins (P- and E-selectins) and endoglin by the EA.hy926 endothelial cell line was similarly investigated. Since no statistically significant changes in the expression of these molecules were observed even after two hours, the time for measuring the dynamics of the process was increased to four hours (Figure 2). However, even increasing the incubation time did not lead to changes in the surface expression of the receptors by the endothelial cells, unlike neutrophil receptors. The fact that endothelial cells were still capable of integrins hyperproduction was evidenced by the fact that in response to the positive control tumor necrosis factor (TNF), endothelial cells increased the expression of both adhesins (VCAM-1 and ICAM-1) and selectins (P- and E-selectins) within an hour (Figure 2).
–
Blocking antibody studies showed that all studied receptors participate very actively in the interaction between neutrophils and the endothelial cell, since in the case of blocking all receptors, as well as blocking all receptors with the exception of one class causes a statistically significant decrease in the work of adhesion (Table 1). VCAM-1, P- and E-selectin receptors contributed most to the interaction between endothelial cells and neutrophils, whereas ICAM-1 had no significant effect on the interaction in the endotheliocyte-neutrophil system. Endoglin could also modulate the total adhesion between neutrophils and endotheliocytes. However, even in the case of complete blockade of all receptors, residual interaction between neutrophils and endothelial cells was observed due to nonspecific interactions (Table 1).
Table 1. Adhesion work as result of specific (receptor-dependent) and non-specific interaction between neutrophil and endothelial cell.
|
Control (all receptors free, not blocked) (n=150) |
Blocked all receptors (ICAM-1, VCAM-1, P- and E-selectin, endoglin) (n=150) |
Blocked all receptors except ICAM-1 (n=150) |
Blocked all receptors except VCAM-1 (n=150) |
Blocked all receptors except P- and E-selectins (n=150) |
Blocked all receptors except endoglin (n=150) |
|---|---|---|---|---|---|
|
324.83 ± 93.73 |
79.88 ± 54.84* |
200.94 ± 52.98* |
262.03 ± 74.77* |
202.54 ± 64.51* |
270.97 ± 81.88* |
*The samples of the control and experimental groups are statistically significantly different, paired t-test (p < 0.05).
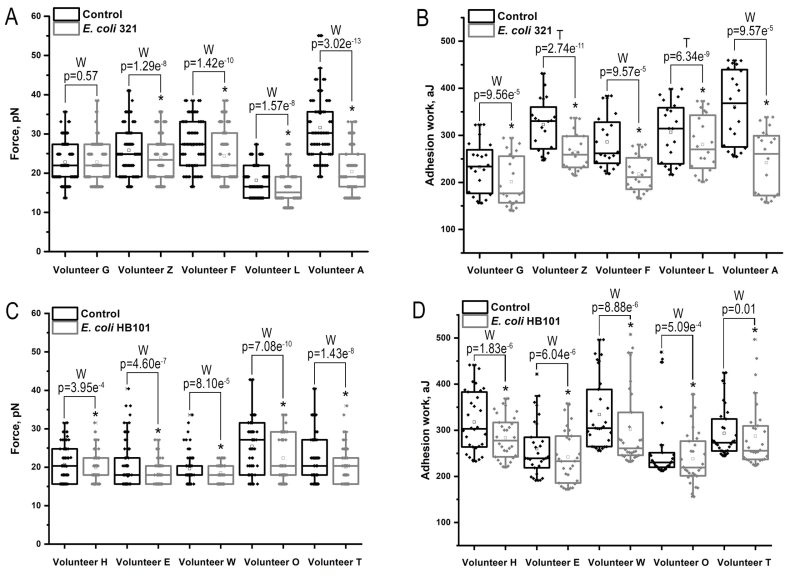
As there was no statistically significant change in surface receptor expression after one hour of E. coli exposure to neutrophils and endothelial cells, we measured adhesion force and adhesion work for 60 minutes. Previously, we had found significant variability in the adhesion activity of neutrophils depending on the cell donor, so we performed pairwise measurements of adhesion force and work in a series of control (neutrophil-endothelial cell interaction) and experimental (neutrophil-endothelial cell interaction after introduction of E. coli 321 into the system) experiments 7. A non-pathogenic strain of E. coli HB 101 was used as an additional control. The results of the study are shown in Figure 3.
–
The results show that in almost all cases there was a statistically significant decrease in the efficiency of adhesive contacts between immune cells when exposed to E. coli 321, as both adhesion force and work were reduced, with only one of the donors showing no change in adhesion force after incubation with the bacteria. However, this property was not unique for pathogenic strain only, since the non-pathogenic strain statistically significantly reduced both the force and work of adhesion (Figure 3, Table 2).
Similar data were obtained when the total force and work of adhesion were studied in all series of experiments (Table 2).
Table 2. E. coli 321 and E. coli HB101 reduce the efficiency of adhesive contacts in the neutrophil-endothelial cell system.
|
|
Adhesion Force, pN |
Adhesion Work, aJ |
|---|---|---|
|
Control (not treated cells) |
25.33 ± 7.96 (n = 350) |
301.59 ± 73.53 (n = 100) |
|
After incubation with E. coli 321 |
22.07 ± 7.22* (n = 350) |
241,03 ± 59.48* (n = 100) |
|
After incubation with E. coli HB101 |
19.53 ± 4.29* (n = 350) |
270.55 ± 68.76* (n = 100) |
* The samples of the control and experimental groups are statistically significantly different, Paired Sample Wilcoxon Signed Ranks test (p<0.05)
The decrease in the efficiency of adhesive contacts could also be due to changes in the mechanical properties of the endothelial cells. Therefore, the stiffness of endothelial cells under the influence of E. coli was investigated. The stiffness of endotheliocytes at points on the cell surface under the influence of E. coli 321 was investigated by FS spectroscopy and the Young’s modulus was calculated using the Hertz model. The results are shown in Table 3.
Table 3. Young’s modulus of endothelial EA.hy926 cells.
|
|
Young’s modulus, kPa |
|---|---|
|
Control (not treated cells) (n=120) |
16.13 ± 3.96 kPa |
|
After incubation with E. coli 321 (n=120) |
18.39 ± 5.19 kPa * |
|
After incubation with E. coli HB101 (n=120) |
16.81 ± 4.11 kPa |
* The samples of the control and experimental groups are statistically significantly different, paired t-test (p<0.05).
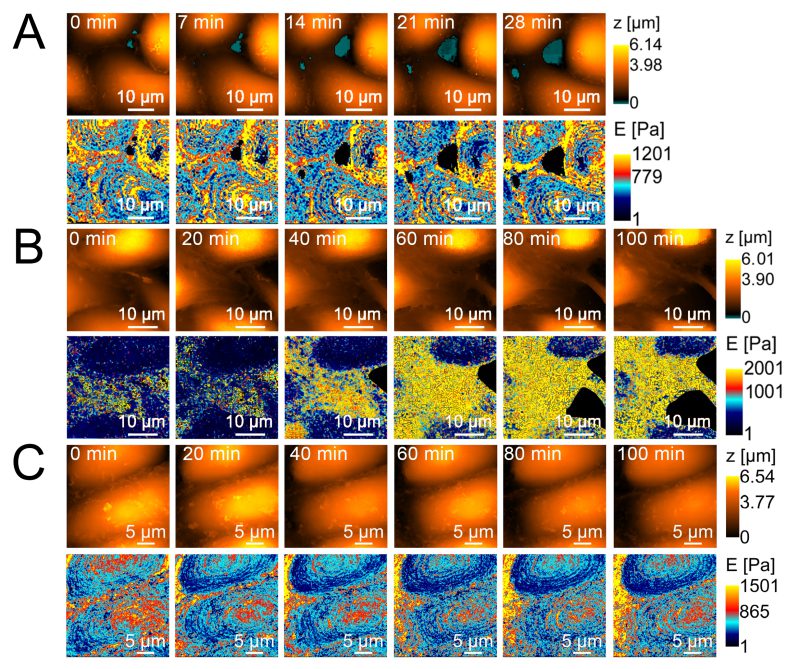
Similar results were obtained when cell stiffness was examined using SICM, which, in contrast to AFM point values, provides a stiffness map. However, in cause with non-pathogenic strain E. coli HB101 no changes in the rigidity of endothelial cells after incubation with the strain were observed. Figure 4 shows the dynamics of changes in endothelial cell stiffness in the control (long-term observation of cells in Hanks’ medium stabilized with 2 mM glutamine and 10 mM HEPES at pH 7.3) and in the experiment after the introduction of 200 μl of E. coli 321 at a concentration of 109 cells/ml.
–
Despite a significant increase in endotheliocyte stiffness after treatment with E. coli 321, cell viability did not change significantly: according to flow cytometry results, the number of viable cells in the control (endothelial cells without exposure) was 99.62 ± 0.04% and after 120 min of co-incubation with E. coli 321 109 cells/ml 98.57 ± 0.10 (Figure 5).
–
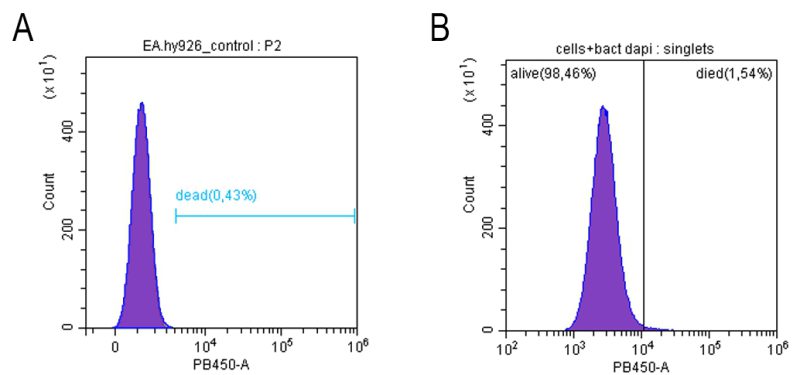 | FIGURE 5: Viability of endothelial cells (cell line Ea.hy926) before (A) and after (B) incubation with E. coli 321 during 120 min. |
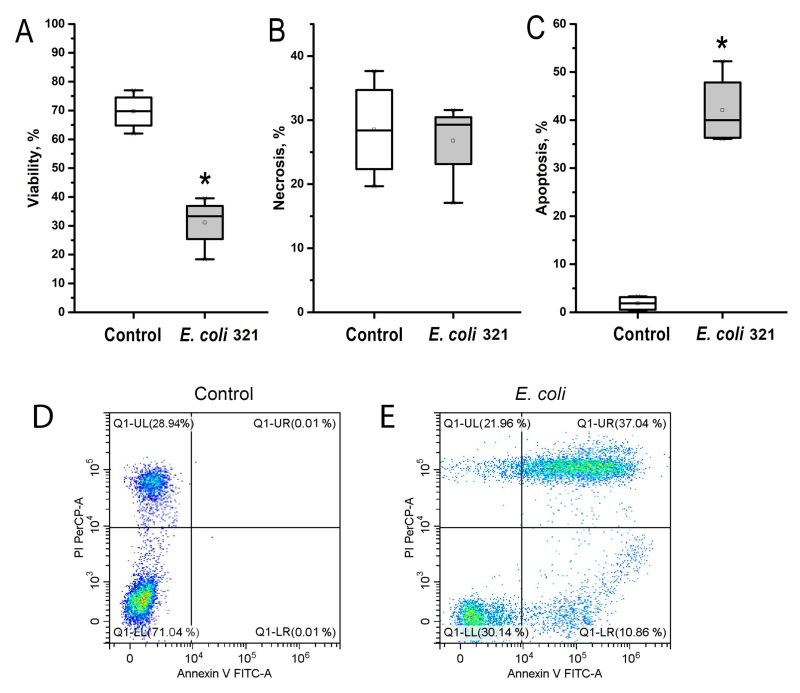
Similar to the results obtained in the surface integrins expression study, neutrophils were much more sensitive to E. coli 321 exposure than endotheliocytes. Figure 6 shows that there was a statistically significant decrease in neutrophil viability as a result of bacterial exposure. In Figures 6 D, E in the flow cytometry dot plots, the upper left quadrant indicates Annexin V negative, PI positive (Annexin V-, PI+) cells, defined as necrotic cells. Upper right quadrant indicates Annexin V positive, PI positive (Annexin V+, PI+) cells, defined as late apoptotic cells. Lower left quadrant indicates Annexin V negative, PI negative (Annexin V-, PI-) cells, defined as viable cells. Lower right quadrant indicates Annexin V positive, PI negative (Annexin V+, PI-) cells, defined as early apoptotic cells. Control indicates that cells in this group received no treatment. Figure 6C shows the percentage of apoptotic cells, which is the sum of the percentages of cells in the upper right and lower quadrants. Evaluation of the levels of necrosis and apoptosis showed that neutrophils under the influence of E. coli 321 died predominantly by the apoptotic mechanism, whereas the level of necrotic death of neutrophils was not significantly different from the control.
–
It was interesting to find that neutrophils also died predominantly by apoptosis and necrosis in another model of inflammation, septicopyemia, when E. coli was used as a chemoattractant in the lower chamber and neutrophils migrated from the upper chamber along a chemoattractant gradient (Figure 7).
–
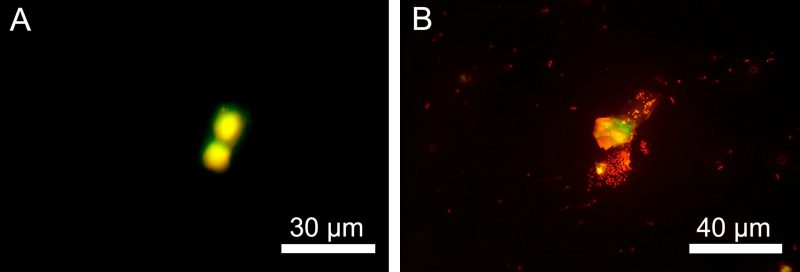 | FIGURE 7: Apoptotic (A) and necrotic (B) death of neutrophil granulocytes in a model of septicopiemia as cells migrate along the E. coli 321 chemoattraction gradient to the lower chamber. |
DISCUSSION
The pathogenic strain E. coli 321 induces activation of neutrophils. The formation of an activation phenotype by neutrophils is evidenced by increased surface expression of CD11a and CD11b receptors. Similarly, in an in vivo model 10 it was shown that neutrophils, regardless of their site of localization (blood, spleen), acquired an activated phenotype with CD11b hyperexpression upon capture of E. coli. Activation and hyperexpression of integrins indicate the physiological readiness of neutrophils for migration processes. In addition, it has been shown by Castillo et al. 11 that RNA isolated from E. coli promotes ICAM-1 expression on HUVEC endothelial cells and also causes activation, adhesion and chemotaxis of neutrophils.
However, firstly, in the septicemia model, the bacteria are already in the bloodstream, and secondly, the results show a significant decrease in the total force and work of adhesion interaction between neutrophils and endotheliocytes in the presence of E. coli. Our studies show that on the endothelial cell line EA.hy926 all types of studied receptors ICAM-1, VCAM-1, P- and E-selectin and endoglin participate very active in the interection between endothelial cell and neutrophil (Table 1). Therefore, both adhesins and selectins may contribute to the reduction of adhesive interactions in response to E. coli invasion. A role for endoglin cannot be excluded either, as it has been shown in blocking antibody experiments that endoglin can also have an effect on adhesion contacts between neutrophils and endotheliocytes. Similar results were obtained by Ruiz-Remolina et al. 12, where it was shown that an increase in the level of soluble endoglin compared to membrane-bound endoglin decreases the expression of ICAM1, VCAM1 and E-selectin during the induction of inflammatory processes. Transendothelial migration of neutrophils same time is a complex, multi-step process that includes: transition to the marginal pool, rolling, activation, adhesion, transmigration and subsequent migration through the extracellular matrix 13. Thus, neutrophil adhesion to endothelial cells is one of the key events in the inflammatory process. However, our experiments showed a failure of the neutrophil adhesion strategy in the case of E. coli alteration. In all donors, both adhesion force and work were reduced under the influence of bacteria. At the same time, the work of adhesion proved to be a more sensitive parameter, whereas the force of adhesion contacts changed less, which may be an indirect evidence for the fact that conformational changes in the receptors of neutrophils and endotheliocytes are less likely than the loss of these receptors or a non-specific shielding of the interaction by bacterial cells.
In previous studies 7 we have shown by Western blot that there is no shedding of integrin and selectin receptors from the membrane of immune cells, so it is most likely that adhesive contacts between neutrophils and endothelial cells are weakened by the shielding effect of E. coli. This assumption is also supported by two facts revealed during the study: (1) the level of receptor expression does not change (Figure 2), although the interaction between immune cells receptors is significantly weakened, (2) the weakening of the interaction occurs not only in the case of using a pathogenic strain E. coli 321, but also non-pathogenic strain of E. coli HB101 (Table 2).
The pathogenic strain E. coli 321 is not only able to mask the adhesive contacts between the endothelial cell and neutrophil, but also to exert a more complex effect on the cells, which was shown by experiments aimed at studying the stiffness of endothelial cells. In particular, over time, the stiffness of endothelial cells increases and their morphology partially changes: the cells become smaller, the cytoplasm becomes thinner and the cytoplasm concentrates around the nucleus. The increase in the stiffness of the membrane-cytoskeleton complex (the Young’s modulus calculation takes into account the resistance not only of the membrane but also of the cytoskeleton) may also indicate conformational changes in adhesion molecules, since these are transmembrane proteins associated with the cytoskeleton. Unlike the pathogenic strain E. coli 321, the nonpathogenic strain E. coli HB101does not cause changes in either rigidity (Table 3) or cell morphology (Figure 4). Taking into account the analyzed genome of the pathogenic strain E. coli 321, adhesins and exotoxin can primarily claim the role of a factor contributing to changes in the morphology and rigidity of endothelial cells.
However, in our opinion, the greatest impact on the decrease in adhesive contact force is exerted by neutrophil death, as a significant decrease in neutrophil viability and their death by the mechanism of apoptosis has been shown. In this case, the initial hyperproduction of integrins CD11a and CD11b can be considered not only as an activation strategy, but also as a compensatory strategy to counterbalance the alteration of the physiological immune cell adhesion response. However, a critical mass of E. coli 321 in our model experiments in vitro or under in vivo conditions leads to neutrophil death by the mechanism of apoptosis and failure of compensation of intercellular adhesion contacts. Here, with a high probability, the pathogenicity factors identified in the process of genotyping are the main adhesins and exotoxin responsible for the neutrophils death.
Thus, in the pathogenesis of sepsis caused by pathogenic strain E. coli 321, it is important to consider not only the death of immune cells and their general morpho-biophysical alteration under the influence of bacteria, but also the disruption of the adhesion strategy of neutrophils during the transition of cells to the marginal pool.
CONCLUSION
In this work, we have demonstrated for the first time that in the model of experimental septicemia there is a disruption of adhesion contacts between neutrophils and endothelial cells, manifested by a decrease in adhesion force and work upon exposure to E. coli. It is shown that this may be due to a mask of receptors by bacterial cells as well as an increase in endothelial cell stiffness under the action of bacteria and neutrophils death by the mechanism of apoptosis under pathogenic strain E. coli influence.
MATERIALS AND METHODS
Work with bacterial culture
Cultivation and standardization of bacterial cells
The strain E. coli 321 was isolated from the blood of a patient with bacteriemia. E. coli 321 and E. coli HB101 strains were grown on solid “GRM” nutrient medium (Obolensk, Russia) then transferred sterile to LB broth (Lennox; Condalab, Madrid, Spain) for incubation (37°C, 24 h). The grown bacterial cells were washed with sterile PBS three times in microtubes (450 g, 5 min). A bacterial count of 10 FTU equivalent was used in all experiments. The optical density of the E. coli suspension in PBS was set to 0.85 (1×109 cells/ml) by a Specs SSP 705 spectrophotometer (Spectroscopic systems, Moscow, Russia) and standardized suspension was used for all future experiments. Routine Gram staining was used to control bacteria morphology. In 14 data are presented that the metabolism of E. coli and its virulence can be determined not only by the media, but also by the growth phase (including adaptation period), however, our task was to ensure maximum viability not only of the bacterial strain, but also of two types of eukaryotic cells (neutrophils and endothelial cells) 15, therefore, the bacteria were immediately transferred to a medium that ensured maximum viability of both bacteria, neutrophils, and endothelial cells – Hanks’ solution contained 2 mM glutamine and 10 mM HEPES.
Strain E. coli 321 genotyping
Bacterial DNA was isolated from an overnight culture using a QIAamp DNA Mini Kit (Qiagen, Düsseldorf, Germany). DNA libraries were prepared using a MGIEasy Uni-versal DNA Library Prep Set (MGI Tech, Shenzhen, China) according to the manufacturer instructions. DNA (300-600 ng) was fragmented using a Covaris S220 ultrasonicator (Co-varis, Woburn, MA). The average fragment length was 250 b.p. Concentrations of DNA and DNA libraries were measured by a Qubit Fluorometer (Thermo Fisher Scientific, USA) with the use of the dsDNA HS Assay Kit according to the manufacturer instructions. The DNA library quality control (determination of the size distribution of the final library and confirmation of the absence of the remaining adapter di-mers) was performed using a Bioanalyzer 2100 and a High Sensitivity DNA kit (Agilent Technologies, Santa Clara, CA). DNA libraries were sequenced on the DNBSEQ-G400 platform in a 100 b.p. pair-end mode with the use of a DNBSEQ-G400RS High-throughput Sequencing Set PE100 kit (MGI Tech, China) according to the manufacturer instructions. The results were analyzed in the FDB Database: VFDB full dataset – all genes related to known and predicted VFs (http://www.mgc.ac.cn/VFs/VFcategory.htm).
Preparation of eukaryotic cells
Isolation of neutrophil granulocytes (NGs) from human blood
Venous blood samples of healthy donors stabilized with heparin (50 IU/ml) were drawn at the Nizhny Novgorod Regional Blood Centre n.a. N.Ya. Klimova. The study was approved by the Bioethics Commission of Lobachevsky State University (established on 11 November 2016, order of establishment No. 497-OD), protocol No. 9 dated 17 July 2017. Whole blood was half diluted with sterile PBS (0.137 M NaCl, 0.0027 M KCl, pH 7.35). Centrifugation of samples was performed on a double Ficoll-Trazograph gradient (ρ = 1.077 g/mL, ρ = 1.110 g/mL, 400 g, 40 min), after that, NGs were taken away and twice washed with sterile PBS (400 g, 3 min) according to 16. Assessed cell viability by propidium iodide staining was 98-99%. The final concentration of NGs in experiments was 1×106 cells/ml.
Probe functionalization and NGs attachment to the cantilever
A C-MSCT Si3N4 cantilever (Bruker, Billerica, MA, USA) with frequency f0 – 4-10 kHz and spring constant k – 0.010 N/m was used for NGs attachment. To improve cell-to-cantilever connection, latter was treated with poly-L-lysine (Sigma-Aldrich, Burlington, MA, USA), then washed three times with sterile PBS (10 µL). Pretreated cantilever was incubated (37°C, 20 min) with 10 µL of NGs suspension then again washed three times with PBS to remove unattached cells. This NGs concentration guaranteed cells fixation on the probe as it was evaluated in the preliminary experiments 17. The microlever with NGs was placed on a NTEGRA AFM (NT-MDT Spectrum Instruments, Moscow, Russia) control head.
EA.hy926 cell culture
The endothelial cells of EA.hy926 line were cultured in Portable Mini NB 203M CO2 incubator (N-Biotek, Bucheon, South Korea) at 37°C and 5% CO2 in DMEM-F12 medium with addition of inactivated fetal calf serum (10%), streptomycin (100 µg/mL), penicillin (100 units/mL), L-glutamine (8 mM), HAT (50 µM hypoxanthine, 0.2 µM aminopterin, 8 µM thymidine) (all – PanEco, Moscow, Russia). Monolayer was detached with 0.25% trypsin-EDTA solution (PanEco, Moscow, Russia). Passages according to the conventional technique 18 were carried out each 3-4 days when the culture reached 90-100% confluency. The monolayer was disaggregated by exposure to trypsin-EDTA solution for 3 minutes. Cells at 3-25 passages were used in the experiments at a 2.5×105 cells/ml concentration to create monolayer in Petri dish (37°C, 5% CO2, 72 h).
Determining the values of force and work of adhesion by AFM-method
In a Petri dish with the endothelial cells (control), the medium was changed to Hanks’ solution contains 2 mM glutamine and 10 mM HEPES (all – PanEco, Moscow, Russia) and then FS spectroscopy was performed.
A cantilever with a neutrophil was exposed above the surface of the endothelial cells using optical microscope for positioning, followed by feedback capture in AFM contact mode. After exposure the cantilever was moved over the endotheliocyte in the height range – 100-5000 nm. The maximum value of the loading force was in the interval of 3-4 nN. The approach-raise time of 2 s, and the probe movement speed of 5 μm/s were used. The interaction force was set in the range of 10-50% of the maximum value. After obtaining the FS-curve, the feedback was switched off to prevent cell damage and a new site of Petri dish with endothelial cells was investigated.
The design of the septicemia model experiment was the same, but E. coli 321 culture at a final concentration of 2.5×107 cells/ml was added to a Petri dish containing endothelial cells prior to adhesion force and work measurements and incubated (25°C, 30 min). In this case, the bacteria were between endothelial cells and neutrophils, reproducing the situation of sepsis and influencing the ligand-receptor interactions between endotheliocytes and neutrophils.
The total force of the adhesive contacts in system with two types of cells was calculated according to following equation:
–
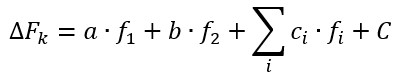 |
The rupture force F is the difference between the applied force before (Fp) and after (Fa) of the receptor bond rupture, these values were obtained experimentally from the Fs curves:
–
The work of adhesion was estimated using the equation:
–
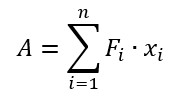 |
where A – work of adhesion, Fi and xi – force and distance measured at the i-point of the Fs-curve.
Determining the values of adhesion force and work after addition of blocking antibodies
To determine which receptors were most involved in the intercellular interaction, blocking antibody studies were performed. The growth medium was removed from the experimental dish and 1 ml of growth medium containing a cocktail of monoclonal antibodies (5 μg/ml) was added to the endotheliocytes: (1) anti-CD62E (5 µg/ml, binds specifically to human E-selectin and P-selectin, Millipore, Burlington, MA, USA), (2) anti-endoglin (5 µg/ml, CCC, Wuhan, PRC) (FITC-conjugated antibodies against mouse IgG (1:1000 dilution, Sigma-Aldrich, Burlington, MA, USA)), (3) phycoerythrin (PE)-conjugated anti-VCAM-1 (0.25 µg/ml, eBioscience, San Diego, CA, USA) and (4) fluorescein isothiocyanate (FITC)-conjugated (1:1000 dilution) anti-ICAM-1 (5 µg/ml, Invitrogen, Waltham, MA, USA). Cells were incubated with antibodies for 30 min (37°C, 5% CO2), washed three times with Hanks’ solution (2 mM glutamine, 10 mM HEPES), FS spectroscopy was performed and adhesion force and work were determined.
A series of experiments were then carried out in which one type of antibody was excluded from the cocktail in turn.
Analysis of adhesion molecule surface expression on neutrophils and endothelial cells exposed to E. coli 321
Flow cytometry was used to assess the key selectin and integrin molecules on endothelial cells (VCAM-1, ICAM-1, P- and E-selectins and endoglin (ENG)) and neutrophils (CD11a/CD18 and CD11b/CD18) after activation by E. coli 321.
Ea.hy926 cells were seeded (5×105 cells/ml) in a 24-well plate and grown in a CO2 incubator (1-4 h, 37 °C, 5% CO2) until a confluent monolayer was obtained (Corning Inc., Corning, NY, USA). A suspension of E. coli 321 (2.5 × 107 cells/mL) or tumor necrosis factor (TNF) at 25 ng/mL (positive control) was then added. Cells were then harvested from the culture plate using 0.25% trypsin EDTA and rabbit anti-human endoglin antibodies (5 µg/test, CCC, Wuhan, PRC) (FITC-labelled goat anti-rabbit IgG antibodies (Sigma-Aldrich, Burlington, MA, USA) were used as secondary antibodies at 1:1000 dilution), anti-CD62E (clone 1. 2B6) specific for human E-selectin and P-selectin (2 µg/test, Millipore, Burlington, MA, USA), anti-VCAM-1 (clone 429) labelled with phycoerythrin (PE) (0.25 µg/test, eBioscience, San Diego, CA, USA) and anti-ICAM-1 (clone MEM-111) labelled with fluorescein isothiocyanate (FITC) (5 µg/test, Invitrogen, Waltham, MA, USA) at 1:1000 dilution were used to evaluate the expression levels of the corresponding adhesion molecules on the EA. hy926 cells surface in the untreated state and after exposure to E. coli 321 and TNF.
Neutrophils were isolated according to the procedure described in section 5.2.1. and then incubated with a suspension of E. coli 321 (2.5 × 107 cells/mL). The cells then were labeled with rabbit anti-human CD11a, CD11b and CD18 (5 µg/test, all from CCC, Wuhan, PRC) (FITC-labelled goat anti-rabbit IgG antibodies (Sigma-Aldrich, Burlington, MA, USA) at 1:1000 dilution were used as secondary antibodies) antibodies to measure the expression levels of the corresponding integrins on the surface of neutrophils.
Mean fluorescence intensity (MFI) values for each antibody expression were evaluated using Cytoflex S flow cytometer (Beckman Coulter, Brea, CA, USA) and CytExpert software v.1.2 (Beckman Coulter, USA).
Scanning ion-conductance microscopy for cell observation and stiffness measurement
EA.hy926 cells in Petri dish were scanned by a scanning ion-conductance microscope (ICAPPIC Ltd., UK) using borosilicate glass nanopipettes as probes. Nanopipettes with a characteristic inner tip radius up to 50 nm were made from B-10050-10F glass capillary with filament (RWD, Shenzhen, PRC) on a P-2000 laser puller (Sutter Instruments, Novato, CA, USA). A hopping mode scanning was performed with a nanopipette fall rate of 100 nm/ms at a bias potential of 200 mV. A MultiClamp 700 B amplifier (Molecular Devices, Wokingham, UK) was used for the ion current measurement. The stress induced on a cell by the nanopipette was evaluated from the gap size in terms of the ion current drop at 0.5% and 2% set points. SICMImageViewer software (ICAPPIC Ltd., UK) was used for image processing and stiffness data evaluation.
Statistical analysis
Origin 8.0 software (Origin Lab Corporation, Northampton, MA, USA) was used for all statistical analyzes. The Shapiro-Wilk test was used to determine the limits of normal distribution for the quantitative indicators of the samples. The mean and standard deviation of data as well as pair Student’s t-test for statistically significant differences evaluation were used when data were normally distributed. Paired Sample Wilcoxon Signed Ranks test, Kruskal-Wallis H test and Mann-Whitney U test were used to compare groups when data were not normally distributed.
ACKNOWLEDGMENTS
The work was supported by Russian Science Foundation Project No 22-14-20001.
The authors express their sincere gratitude to Prof. S.A. Selkov, Head of the Department of Immunology and Intercellular Interactions, and Dr. D.I. Sokolov, Head of the Laboratory of Intercellular Interactions, D.O. Ott Research Institute of Obstetrics, Gynaecology and Reproduction, for providing the EA.hy926 cell line. Ott Research Institute of Obstetrics, Gynecology and Reproductology for providing the EA.hy926 cell line; Dr A.S. Erofeev, Head of the MISIS Biophysics Laboratory, and V.S. Kolmogorov, Laboratory Engineer, for providing nanopipettes.
COPYRIGHT
© 2024

Pathogenic Escherichia coli change the adhesion between neutrophils and endotheliocytes in the experimental bacteremia model by Pleskova et al. is licensed under a Creative Commons Attribution 4.0 International License.