Reviews:
Microbial Cell, Vol. 3, No. 9, pp. 371 - 389; doi: 10.15698/mic2016.09.524
Gonorrhea – an evolving disease of the new millennium
Department of Biological Sciences, Northern Illinois University, DeKalb, IL 60115 USA.
Keywords: pathogenesis, antigenic variation, immune manipulation, antibiotic resistance, panmictic.
Received originally: 09/10/2015 Received in revised form: 28/01/2016
Accepted: 30/01/2016
Published: 05/09/2016
Correspondence:
Stuart A. Hill, Tel: (815) 753-7943; Fax: (815) 753-7855 sahill@niu.edu
Conflict of interest statement: The authors declare no conflict of interest.
Please cite this article as: : Stuart A. Hill, Thao L. Masters and Jenny Wachter (2016). Gonorrhea – an evolving disease of the new millennium. Microbial Cell 3(9): 371-389.
Abstract
Etiology, transmission and protection: Neisseria gonorrhoeae (the gonococcus) is the etiological agent for the strictly human sexually transmitted disease gonorrhea. Infections lead to limited immunity, therefore individuals can become repeatedly infected. Pathology/symptomatology: Gonorrhea is generally a non-complicated mucosal infection with a pustular discharge. More severe sequellae include salpingitis and pelvic inflammatory disease which may lead to sterility and/or ectopic pregnancy. Occasionally, the organism can disseminate as a bloodstream infection. Epidemiology, incidence and prevalence: Gonorrhea is a global disease infecting approximately 60 million people annually. In the United States there are approximately 300, 000 cases each year, with an incidence of approximately 100 cases per 100,000 population. Treatment and curability: Gonorrhea is susceptible to an array of antibiotics. Antibiotic resistance is becoming a major problem and there are fears that the gonococcus will become the next “superbug” as the antibiotic arsenal diminishes. Currently, third generation extended-spectrum cephalosporins are being prescribed. Molecular mechanisms of infection: Gonococci elaborate numerous strategies to thwart the immune system. The organism engages in extensive phase (on/off switching) and antigenic variation of several surface antigens. The organism expresses IgA protease which cleaves mucosal antibody. The organism can become serum resistant due to its ability to sialylate lipooligosaccharide in conjunction with its ability to subvert complement activation. The gonococcus can survive within neutrophils as well as in several other lymphocytic cells. The organism manipulates the immune response such that no immune memory is generated which leads to a lack of protective immunity.
INTRODUCTION
Neisseria gonorrhoeae (the gonococcus) is a Gram-negative diplococcus, an obligate human pathogen, and the etiologic agent of the sexually transmitted disease, gonorrhea. The gonococcus infects a diverse array of mucosal surfaces, some of which include the urethra, the endocervix, the pharynx, conjunctiva and the rectum [1]. In 2013, the Centers for Disease Control and Prevention (CDC) reported that there were 333,004 new cases of gonorrhea in the United States, with an incidence of 106.1 cases per 100,000 population [2]. Worldwide, 106.1 million people are infected by N. gonorrhoeae annually [3]. In most cases, the disease is a noncomplicated mucosal infection. However, in a few patients, generally with women, more serious sequelae can occur and include salpingitis (acute inflammation of the fallopian tubes), pelvic inflammatory disease (PID; an infection in the upper part of the female reproductive system), or, in rare cases, as a bacteremic infection [4]. If left untreated, these more serious complications can result in sterility, ectopic pregnancy, septic arthritis, and occasionally death. Approximately 3% of women presenting with a urogenital infection develop the most severe forms of the disease [5]. However, the occurrence of PID has significantly decreased over time [6][7][8], with an estimated 40,000 cases of infertility in women annually [9]. Dissemination rarely occurs, but when the bacteria do cross the endothelium, they can spread to other locations in the body. Currently, a more worrying trend has emerged, in that, there now appears to be an increased risk for HIV infection in patients that are also infected with N. gonorrhoeae [10].
–
Gonorrhea the disease was initially described approximately 3,500 years ago, but it was not until 1879 that Albert Neisser determined the etiologic agent of the disease [11]. The Neisseriae are usually regarded as microaerophilic organisms. However, under the appropriate conditions, they are capable of anaerobic growth [12]. In vitro cultivation of this fastidious organism has always been problematic and it was not until the development of an improved Thayer-Martin medium that early epidemiological studies could be undertaken. Subsequently, other commercial growth mediums have since been developed which has allowed for a greater understanding of the disease process.
VIRULENCE FACTORS OF N. GONORRHOEAE
Like many Gram-negative bacterial pathogens, N. gonorrhoeae possesses a wide range of virulence determinants, which include the elaboration of pili, Opa protein expression, lipooligosaccharide expression (LOS), Por protein expression and IgA1 protease production that facilitates adaptation within the host.
–
Type IV pili (Tfp)
Considerable attention was paid to pili stemming from the observations of Kellogg and coworkers [12][13] that virulent (T1, T2 organisms) and avirulent (T3, T4 organisms) strains could be differentiated on the basis of colony morphology following growth on solid medium. Subsequently, it was established that all freshly isolated gonococci possessed thin hair-like appendages (pili) which were predominantly composed of protein initially called pilin but subsequently renamed PilE [14]. The elaboration of pili is a critical requirement for infection as this structure plays a primary role in attaching to human mucosal epithelial cells [15], fallopian tube mucosa [16][17], vaginal epithelial cells [16][18] as well as to human polymorphonuclear leukocytes (PMN’s; neutrophils) [19][20]. Due to their prominent surface location, pili were initially thought to be an ideal vaccine candidate as pilus-specific antibodies were observed in genital secretions [18]. However, two prominent vaccine trials failed, with evidence indicating that pilus protein(s) underwent antigenic variation [21].
–
Gonococcal pili are categorized as Type IV pili, as the PilE polypeptide is initially synthesized with a short (7 amino acid) N-terminal leader peptide, which is then endo-proteolytically cleaved [22]. The mature PilE polypeptide is then assembled at the inner membrane into an emerging pilus organelle with the PilE polypeptides being stacked in an α-helical array [23]. The PilE polypeptide consists of three functional domains based on sequence characteristics [24]. The N-terminal domain is highly conserved and is strongly hydrophobic, with this region of the protein comprising the core of the pilus structure [23]. The central part of the PilE monomer is partially conserved and structurally aligned as a β-pleated sheet. As the C-terminal domain is hydrophilic, this segment of the protein is exposed to the external environment [23] and undergoes antigenic variation which allows the bacteria to avoid recognition by the human host’s immune cells (reviewed [25][26]).
–
Assembly of the pilus structure is complicated and involves other proteins besides PilE (e.g., the pilus tip-located adhesion, PilC) [27] as well as other minor pilus components PilD, PilF, PilG, PilT, PilP and PilQ [28]. During pilus biogenesis, and prior to assembly, the leader peptide is removed from PilE by the PilD peptidase [23]. The N-terminal domain then facilitates translocation across the cytoplasmic membrane allowing PilE subunits to be polymerized at the inner membrane [29][30]. As the pilus structure is assembled, it is extruded to the exterior of the outer membrane using the PilQ pore forming complex [29][30][31]. PilC is a minor protein located at the tip of pilus as well as being present at its base. The pilC gene exists as 2 homologous, but non-identical copies, pilC1 and pilC2 in most gonococcal strains, with only the pilC2 gene being expressed in piliated N. gonorrhoeae MS11 strains [27]. pilC expression is also subject to RecA-independent phase variation (on/off switching) due to frequent frameshift mutations occurring within homo-guanine tracts located within its signal peptide region [27]. PilC participates in pilus biogenesis as well as in host cell adherence, as pilC mutants prevent the formation of pili by negatively affecting their assembly process, which leads to the bacteria being unable to adhere to human epithelial cells [32].
–
In addition to promoting attachment to host cells, type IV pili are also involved in bacterial twitching motility, biofilm formation, and DNA transformation [33]. N. gonorrhoeae is naturally competent for transformation in that it can take up exogenously produced Neisseria-specific DNA containing a 10-bp uptake sequence (GCCGTCTGAA; DUS) [34]. pilE mutations resulting in loss of pilus expression lead to transformation incompetence [28][35]. The binding and uptake of exogenous DNAs by N. gonorrhoeae requires type-IV-pili-structurally-related components, including ComP protein [36][37]. Despite sharing sequence similarity to PilE in the N-terminal domain, ComP was shown to be dispensable to Tfp biogenesis [36]. Instead the bacteria were unable to take up extraneous DNA; subsequent overexpression of ComP increased sequence-specific DNA binding, suggesting that ComP functions in the DNA binding step of transformation [37]. Recently, ComP has been shown to preferentially bind to DUS-containing DNAs via an electropositive stripe on its surface [38] with uptake of the DNA being facilitated by de-polymerization of the pilus structure through PilT hydrolytic activity [39]. The coordinated physical retraction and elongation of pili can lead to “twitching”, a form of motility that propels the cell along a surface. Retraction is facilitated by PilT activity (an ATPase), whereas PilF protein promotes pilus elongation at the inner membrane [39][40].
–
Por protein
The outer membrane porin protein, Por, is the most abundant protein in the gonococcus accounting for approximately 60% of the total protein content [1]. The molecular size of Por varies between strains, yet, within individual strains, it exists as only a single protein species [41]. Por has been used as the basis for serological classification of gonococci [41] with nine distinct serovars being identified [42]. Overall, there are two distinct structural classes (PorA and PorB) [42], with the PorA subgroup tending to be associated with the more complicated aspects of the disease, whereas the PorB subgroup is more likely to be involved with uncomplicated mucosal infections [43].
–
Porins allow the transport of ions and nutrients across the outer membrane and can also contribute to the survival of the bacteria in host cells [44]. Moreover, gonococcal Por protein has been shown to translocate from the outer membrane into artificial black lipid membranes [45] as well as into epithelial cell membranes, following attachment of the bacteria [46]. Por can also transfer into mitochondria of infected cells which leads to the formation of porin channels in the mitochondrial inner membrane, causing increased permeability [47]. This causes the release of cytochrome c and other proteins, leading to apoptosis of infected cells [48]. However, Por-induced apoptosis remains controversial. In direct contrast to events with the gonococcus, Neisseria meningitidis Por, which also interacts with mitochondria, apparently protects cells from undergoing apoptosis [49]. Interestingly, mitochondrial porins and Neisseria PorB share similar properties, with both protein species being capable of binding nucleotides and exhibiting voltage-dependent gating [50]. Por protein also modulates phagosome maturation by changing the phagosomal protein composition through the increase of early endocytic markers and the decrease of late endocytic markers, which ultimately delays phagosome maturation [51].
–
Opacity-associated protein (Opa)
Opa proteins are integral outer membrane proteins and cause colonies to appear opaque due to inter-gonococcal aggregation when viewed by phase-contrast microscopy [52][53][54]. Opa proteins belong to a multigene family with a single gonococcal cell possessing up to 12 opa genes that are constitutively transcribed [55][56]. Each gene contains conserved, semivariable and 2 hypervariable regions, with the hypervariable segments of the proteins being located on the outside of the outer membrane [55]. Opa protein expression can undergo phase variation due to changing the numbers of pentameric repeat units (-CTCTT-) that are located within the leader peptide encoding region, which results in on/off switching of expression [57]. A single cell is capable of expressing either none to several different Opa proteins [57][58].
–
Unlike pili, Opa expression is not required for the initial attachment of gonococci to the host. However, as an infection proceeds, Opa expression varies [58], and Opa-expressing bacteria can be observed in epithelial cells and neutrophils upon re-isolation from infected human volunteers [59][60]. The invasive capacity of N. gonorrhoeae is determined by the differential expression of Opa [61]. Individual Opa proteins bind to a variety of receptors on human cells through their exposed hypervariable regions. The binding specificity for human receptors falls into two groups: OpaHS which recognize heparin sulfate proteoglycans [62][63]; and, OpaCEA which recognize the carcinoembryonic antigen cell adhesion molecule (CEACAM) family that is comprised of the various CD66 molecules [64][65][66][67]. CEACAMs are the major receptors of Opa proteins and are expressed on many different cell types including epithelial, neutrophil, lymphocyte and endothelial cells [68].
–
Lipooligosaccharide (LOS)
As with all Gram-negative bacteria, gonococci possess lipopolysaccharide in the outer membrane. Gonococcal LPS is composed of lipid A and core polysaccharide yet lacks the repeating O-antigens [1]. Accordingly, gonococcal LPS has been designated as lipooligosaccharide (LOS). Due to its surface exposure, gonococcal LOS is a primary immune target along with the major outer membrane protein Por [69][70][71]. Gonococcal LOS is also toxic to fallopian tube mucosa causing the sloughing off of the ciliatory cells [72]. The LOS oligosaccharide composition is highly variable both in length and in carbohydrate content. Consequently, heterogeneous LOS molecules can be produced by a single cell. However, distinct forms of LOS may be a prerequisite for infection in men [73]. The most common carbohydrates associated with isolated LOS molecules are lacto-N-neotetraose (Galβ(1-4)GlcNAcβ(1-3)Galβ(1-4)Glc) and digalactoside Galα(1-4)Gal and switching from one form to another occurs at high frequency [74] through phase variation of glycosyl transferases [75] [76]. The variable oligosaccharide portions of LOS can also mimic host glycosphingolipids, thus promoting bacterial entry [74]. In addition, gonococcal LOS can also be sialylated which renders the bacteria resistant to serum killing [77][78][79][80]. Consequently, gonococcal LOS contributes to gonococcal pathogenicity by facilitating bacterial translocation across the mucosal barrier as well as by providing resistance against normal human serum [81][82].
–
IgA protease
Immunoglobin A (IgA) protease is another virulence factor in N. gonorrhoeae [83]. Upon release from the cell, the protein undergoes several endo-proteolytic cleavages, leading to maturation of the IgA protease [84]. During an infection, the mature protease specifically targets and cleaves IgA1 within the proline-rich hinge region of the IgA1 heavy chain. The human IgA2 subclass is not cleaved by gonococcal IgA protease since it lacks a susceptible duplicated octameric amino acid sequence [85]. Neisseria IgA protease also cleaves LAMP1 (a major lysosome associated membrane protein), which leads to lysosome modification and subsequent bacterial survival [86]. Furthermore, iga mutants are defective in transcytosis of bacteria across an epithelial monolayer [87].
PATHOGENESIS
Neisseria gonorrhoeae primarily colonizes the urogenital tract after sexual contact with an infected individual [88]. The gonococcus can exist as both an extracellular and intracellular organism, with the bulk of its genes being devoted to colonization and survival, due to the fact that it cannot survive outside of a human host [89]. Transmission is generally a consequence of sexual intercourse. Upon arrival into a new host, micro-colony formation commences on non-ciliated columnar epithelial cells approximately 1 to 2 hours post-infection [90][91]. Once the micro-colonies achieve a cell density of approximately 100+ diplococci, cytoskeletal rearrangement and host protein aggregation occurs, which leads to pilus-mediated attachment of the gonococcus to the CD46 host cell-surface receptor (Fig. 1) [89][92]. Once bound, the pilus structures on some organisms are retracted through PilE depolymerization [39] which promotes tighter contact with the host cells through Opa binding to the CEACAM receptors (Fig. 1) [65][66]. Upon CEACAM binding, actin polymerization and rearrangement is induced within the host cell which results in bacterial engulfment, transcellular transcytosis and release of the bacteria into the subepithelial layer (Fig. 1) [68][93].
–
In vivo, the coordinated expression of pili and Opa varies considerably [94]. Organisms isolated from the male urethra generally co-express pili and one of several Opa proteins [58]. However, in women, Opa expression varies depending upon the stage of the menstrual cycle and whether or not the patient is taking oral contraceptives [94]. At mid-cycle, bacteria isolated from the cervix express Opa, whereas those isolated during menses tend to be Opa negative [17]. Moreover, organisms isolated from infected fallopian tubes are almost universally Opa negative, even though Opa expressing organism can be isolated from the cervix of the same patient [17]. These observations can perhaps be explained by the fact that cervical secretions during menstruation contain more proteolytic enzymes than during the follicular phase. Consequently, non-Opa expressing cells may be selected due to the extreme sensitivity of Opa proteins to trypsin-like enzymes. However, with the recent studies demonstrating Opa interactions with CECAM receptors, it has been observed that fallopian epithelial tube cell cultures do not appear to express CECAM receptors [95]. Nonetheless, in the absence of these receptors, gonococci were found to still adhere and invade. Consequently, CECAM expression, or the lack of it, possibly allows for in vivo phenotypic selection of distinct gonococcal populations on various tissues [96]. Overall, Opa expression does appear to increase gonococcal fitness within the female genital tract [97]. Generally, Opa expression is absent in most re-isolates from female disseminated infections.
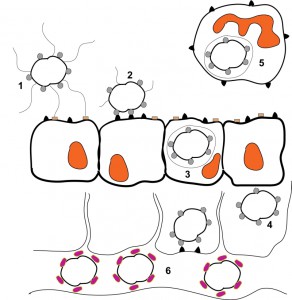 | FIGURE 1: Schematic representation of a Neisseria gonorrhoeae infection. 1) Piliated, Opa-expressing gonococci interact with the mucosal epithelium. The thin, hair-like pilus appendages provide the initial contact with receptors on the surface of the mucosal cells. 2) Pili are then retracted which allows for more intimate, Opa-mediated attachment of the bacteria with the CD66 antigens located on the mucosal cells. 3) Following Opa-mediated attachment, the bacteria are engulfed and are internalized into the mucosal cells. 4) Following internalization, some bacteria can transcytose to the basolateral side of the mucosal epithelium. 5) Depending upon which Opa protein is being expressed, gonococci can also reside and survive inside of neutrophils. 6) Following transcytosis, gonococci can enter the bloodstream where heavy sialylation of lipooligosaccharide renders the bacteria serum resistant. This figure is based on [98]. |
–
Inflammation
The hallmark symptom of a non-complicated gonorrhea infection is a massive recruitment of neutrophils to the site of infection leading to the formation of a pustular discharge. Initially, Opa protein expression was suspected to be intimately involved in PMN stimulation [20][98][99][/cite][100]. Subsequently, it was shown that following attachment of gonococci to the mucosa, the pro-inflammatory cytokines IL-6 and TNF-alpha as well as the chemokine IL-8 are released leading to the recruitment of neutrophils [101]. In addition, upon arrival at the sub-epithelial layer, gonococci release LOS and lipoproteins which further stimulate cytokine production [102] as these outer membrane components are detected by Toll-like receptors (TLRs) on immune cells [103]. Host cells also respond to bacterial peptidoglycan fragments within outer membrane vesicles via cytoplasmic NOD-like receptors (NLRs) which also contribute to the secretion of additional pro-inflammatory cytokines [104].
–
Despite the active recruitment of PMNs to a site of infection, gonococci can survive the oxidative and non-oxidative defense mechanisms (Fig. 1) [105]. Survival appears to correlate with gonococci selectively triggering Th17-dependent host defense mechanisms by modulating expression of IL-17 [106]. Gonococci also must combat considerable oxidative stress by elaborating a number of different enzymes during the inflammatory response in order to detoxify superoxide anions (O2•−), hydrogen peroxide (H2O2), and hydroxyl radicals (HO•) [107][108]. Gonococci must remove H2O2 because in the presence of ferrous ions the Fenton reaction is initiated (Fe2+ + H2O2 → Fe3+ + OH. + OH−) which yields additional hydroxyl radicals [109][110]. Catalase is used by the gonococcus to eliminate H2O2 (which significantly increases the organism’s ability to resist in vitro neutrophil killing) [111] in conjunction with a periplasmic cytochrome c peroxidase (Ccp) [109]. Normally, superoxide ions are removed by superoxide dismutase enzymes (SOD) which convert superoxide to H2O2 and water. However, the majority of N. gonorrhoeae strains have no measurable SOD activity [107][110], suggesting that oxidants may be removed via an alternative mechanism. It appears that N. gonorrhoeae utilize manganese ions (Mn2+) to combat reactive oxygen species accumulation. Manganese accumulates within the cell through the Mn uptake system (MntABC), with Mn(II) and Mn(III) both scavenging superoxide and hydrogen peroxide molecules non-enzymatically. Furthermore, Mn(II)-pyrophosphate and Mn(III)-polyphosphate complexes are also effective in eliminating hydroxyl radicals that are formed via the Fenton reaction [109].
–
The need for iron
Despite the problems associated with the Fenton reaction, iron is a vital nutrient, with pathogens expending considerable resources on scavenging the element from their human host. This becomes even more complicated during an infection, as the host responds to inflammation by limiting iron availability, as well as by decreasing free iron within the bloodstream [112]. Even though humans keep their iron sequestered in iron-protein complexes such as transferrin, lactoferrin, haemoglobin, and ferritin, the Neisseria are capable of scavenging iron from both transferrin and haemoglobin [113], and express receptors for both transferrin and lactoferrin that provide a selective advantage within the host [114]. Because Neisseria do not produce siderophores, they must directly extract iron from transferrin. To achieve this, the iron transport system consists of two large surface proteins, transferrin binding protein A (TbpA) and transferrin binding protein B (TbpB), with both of these proteins being found in all clinical isolates of pathogenic Neisseria [115]. TbpA is an outer membrane transporter essential for iron uptake that binds both apo- and iron-containing transferrin with similar affinities, whereas TbpB, a surface-exposed lipoprotein, only associates with iron-bound transferrin [116]. As the affinity of the bacterial receptor for iron is similar to transferrin’s affinity, this enables the gonococcus to compete with the host for this necessary nutrient [117]. Subsequently, it was shown that the expression of the transferrin receptor was absolutely required for gonococcal infectivity [118].
–
Serum resistance
Bactericidal antibody-mediated killing was found to vary greatly between patients presenting genital infections [119]. Subsequently, it was soon recognized that gonococcal surface components were the primary targets of antibody-dependent complement killing, with LPS-specific antibodies being the most effective at inducing bactericidal responses [120]. Two forms of serum resistance were initially described; stable and unstable serum resistance [77][121]. Unstable serum resistance is due to the modification of gonococcal LOS through the addition of sialic acid molecules to terminal galactose residues using cytidine 5’-monophosphate N-acetylneuraminic acid (CMP-NANA) which is abundant in human serum, as well as in various mucosal secretions and within professional phagocytes. Sialic acid transfer uses the conserved outer membrane-located enzyme 2,3-sialyltransferase [79]. Sialylation of LOS mediates both the entry of gonococci into host mucosal cells as well as influencing bacterial resistance to killing by complement [82]. Gonococcal cells harboring lightly sialylated LOS molecules are able to invade host epithelial cells more efficiently than heavily sialylated-LOS variants. However, lightly sialylated-LOS expressing cells are more susceptible to complement-mediated killing, whereas, heavy sialylation of LOS renders the bacteria resistant to normal human serum by masking the target sites for bactericidal antibodies [78][80] which prevents the functional activation of the complement cascade (Fig. 1) [81].
–
In contrast, stable serum resistance appears to be caused through the faulty insertion of the C5b-C9 membrane attack complex in serum resistant strains [122][123][124]. Accompanying this defect in deposition, blocking antibody is also thought to cause the C3 complement component to be loaded onto a different site on the outer membrane such that it again hinders bactericidal killing [125]. Clearly, complement resistance is important for organisms causing a disseminated infection, but its value is less clear for those organisms causing a mucosal infection. However, seminal plasma does contain an inhibitor of complement activation suggesting that there is some complement activity at the mucosa [126].
–
As indicated previously, the major outer membrane protein, Por, exists in two forms, Por1A and Por1B, with Por1A-expressing gonococci being most often associated with disseminated infections [42][43]. Por1A-expressing gonococci also bind complement factor H more efficiently, and, as factor H down-regulates alternative complement activation, such binding helps explain serum resistance in these disseminated strains [127]. Furthermore, it also helps explain species-specific complement evasion [128]. Por protein also influences activation of the classical complement pathway, as Por binds to the C4b-binding protein, which again down-regulates complement activation [129]. Consequently, as factor H and C4b-binding sites on the Por proteins impede functional complement deposition these may need to be modified in vaccine preparations as this may help alleviate problems associated with serum resistance [130].
–
Active immunity
It has long been known that gonorrhea does not elicit a protective immune response and nor does it impart immune memory. Consequently, individuals can become repeatedly infected. Nonetheless, specific antibodies are generated within the genital tract that inhibit adherence to the mucosal epithelium, yet their persistence appears to be short-lived [18][131]. Overall, the immune response to an uncomplicated genital infection remains modest [132].
–
The general unresponsiveness to an infection appears to stem from the organism being able to manipulate the host cell response. Transient decreases in T-cell populations occur within the bloodstream and appear to reflect Opa protein interactions with CD4+ T-cells which suppresses T-cell activation [133]. Moreover, in contrast to Opa-mediated interactions with CEACAM antigens on other cell types, Opa-CEACAM1 T-cell interactions do not appear to cause the internalization of bacteria into the T-cells. This then leads to a dynamic re-cycling response with the T-cells that ultimately suppresses an immune response [134]. Likewise, Opa-CEACAM1 interactions on B lymphocytes also inhibit antibody production [132] [135]. Even with dendritic cells, Opa-CEACAM1 interactions do not stimulate internalization [135]. Instead, engulfment by dendritic cells is mediated through LOS interaction with DC-SIGN antigens. Consequently, as LOS molecules vary in composition, this allows the gonococcus a further opportunity for immune evasion [136]. LOS molecules often activate immune cells through interaction with Toll-like receptors. However, LOS deacylation can moderate an immune response following interaction with its cognate Toll-like receptor leading to B-cell proliferation where antibody production is down-regulated [137].
–
Recently, an artificial estradiol-induced mouse infection model has been developed for gonococcal infections that allows for in vivo assessment [138]. However, major differences exist between the human and mouse female genital tract. For example, the pH of the mouse vagina is higher, there is no comparable menstral cycle, fewer anaerobic commensal bacteria are present, and as the mice need to be treated with antibiotics, this aspect dramatically changes the resident flora [139]. Nonetheless, the mouse infection model has yielded several interesting observations. Using the model, gonococci have been shown to moderate the murine innate immune response by stimulating IL-17 release from TH17 cells which subsequently effects other cells [106]. In conjunction with transforming growth factor beta (TGF-beta), this coupled cytokine presence suppresses Th1/Th2 adaptive responses [140]. Therefore, as the genital tract is rich in TGF-beta, gonococci naturally inhabit an immunosuppressive environment [141]. Again, LOS and Opa expression play a major role in these responses, as LOS drives the Th17 response with Opa negatively impacting the Th1/Th2 responses [141]. Further manipulation of the host response is also seen with gonococcal activation of IgM-specific memory B-cells in a T-independent manner. Consequently, this elicits a non-specific polyclonal immunoglobulin response without generating specific immunologic memory to the gonorrhea infection [142]. Recently, human CEACAM transgenic mouse models have been developed for studying gonococcal in vivo infections [143][144]. With these more refined models, gonococci were shown to readily infect and cause inflammation in the transgenic animals and that Opa-CEACAM interactions dramatically reduced exfoliation of the murine mucosal surface. As gonococci bind to human CR3 (hCR3) integrin to invade cervical cells and that human factor H bridges the interaction between the bacteria and hCR3, then future transgenic mouse models, expressing both hCR3 and human factor H, may further mimic a bona fide gonococcal infection in vivo.
–
Antigenic variation
Neisseria gonorrhoeae can survive either as an extracellular organism, or, alternatively, as an intracellular organism within a variety of different cell types. Which state the organism enters depends largely on which surface components are expressed and whether these components are chemically modified or not. N. gonorrhoeae can modulate expression, or, the chemical character of its surface components either by phase variation, or, by antigenic variation [25]. Generally, phase variation is a consequence of frame-shifting within a gene which leads to random switching between on/off states, whereas antigenic variation leads to changes in the chemical composition of some structural component. Therefore, each gonococcal cell can differentially express distinct surface antigens, in various chemical forms, which hinders recognition by host antibodies, facilitates multiple lifestyles [25] and helps explain the lack of efficacious vaccines to protect against a gonorrheal infection [21].
–
From genome analysis, 72 putative genes were identified that have the capacity to undergo phase variation [145]. Consequently, the stochastic expression of various surface components leads to the emergence of micro-populations that allows colonization within unique environmental niches [146]. Pilus expression can undergo on/off switching due to frameshifting either within the pilE gene [35], or, within the pilC gene [27]. Similarly, LOS variation depends upon frameshifting within various glycosyl transferase genes which leads to the random acquisition of various sugar moieties on a varying LOS molecule [75][76]. Opa expression relies exclusively on phase variation, as a series of pentameric repeats (-CTCTT-) reside towards the 5′ end of each opa gene [57]. Consequently, the addition or subtraction of a repeat(s) will bring each individual opa gene either in or out of frame. As expression of individual Opa proteins influence the cellular tropism of the organism with regards to internalization into either mucosal or lymphocytic cells, opa phase variation allows variable gonococcal populations to be established that have the potential to internalize into whatever cell becomes available [56][61]. Consequently, phase variation confers a degree of fitness on the organism for a specific environment, yet provides little with respect to bona fide immune evasion.
–
Antigenic variation on the other hand confers remarkable immune evasion. Antigenic variation occurs extensively within the pil system as well as in some other minor systems (maf and fha) [26]. Gonococci possess multiple variable pil genes; some are deemed silent (pilS) and serve as storage loci for variable pil sequence, and act in conjunction with a single expression locus, pilE, which encodes the PilE polypeptide. Recombination frequently occurs between pilE and an individual pilS leading to changes in the chemical composition of PilE. It is estimated that PilE can assume 108 chemical forms [147] which helps thwart an efficacious immune response due to its prominent surface location. Therefore, despite the fact that anti-pilus antibodies can be detected within the genital tract such antibodies do not recognize heterologous strains thus allowing for reinfection of an individual [18].
–
It is in the coordinated variation of these various surface components that allow gonococci to develop adaptive strategies where the organism can exist either externally or internally during an infection (Fig. 1). When gonococci reside externally, the organisms are generally piliated, with PilE undergoing antigenic variation which negates the various antibody clearing strategies. When coupled with the appropriate LOS composition, these organisms can also become heavily sialylated, which impedes serum killing, thus facilitating extra-cellular growth. In contrast, internalization into host cells requires the retraction of pili causing the cells to become non-piliated. When coupled with phase variation of Opa expression and a non-sialylatable LOS phenotype, the gonococcus can translocate across the mucosal epithelium at an initial stage of the infection and ultimately reside internally within various cell types [25]. Eventually, infected host cells will undergo apoptosis, releasing bacteria back onto the mucosal lining, where in the presence of seminal plasma the appropriate cell surface reappears to facilitate transit into a new host [148].
–
Vaccine development
Vaccine development for sexually transmitted diseases has long been a goal of the scientific community [149][150]. However, given the extensive antigenic variation displayed by N. gonorrhoeae, coupled with suppression and manipulation of the host immune response, progress has been severely impeded. Nonetheless, in the mouse infection model, if Th1 responses can be induced, an infection will clear and immune memory can be established [151]. Consequently, incorporating Th1-inducing adjuvants within any vaccine preparation may be crucial for success in this endeavor.
–
Two outer membrane proteins have come under considerable scrutiny as potential vaccine components; pilus constituents and the major outer membrane protein, Por. Because anti-pili antibodies were detected in vaginal secretions following an infection [18], this led to the early development of a parenteral pilus vaccine. Unfortunately, administration of this vaccine afforded partial protection only to homologous strains. Moreover, it also showed poor immunogenicity and did not stimulate an adequate antibody response at the site of infection [21][152][153]. Consequently, other antigens were explored as potential vaccine candidates. As neisserial Por proteins can serve as adjuvants to B-cells, as well as stimulate Por-specific circulating Th2-cells that appear to migrate to mucosal surfaces, Por has come under considerable scrutiny [154][155]. Por is also capable of stimulating dendritic cells where activation depends on Toll-like receptor 2. Therefore, as Por composition is relatively stable, this protein has become a promising vaccine candidate, especially if Th1-inducing adjuvants and Toll-like 2-inducing adjuvants can be included within any “designer” vaccine preparation [156][157][158].
–
However, a problem exists in the development of any vaccine in that antibodies within normal human serum bind to the gonococcal outer membrane protein Rmp with binding apparently, having important consequences with regard to serum resistance for the organism [159][160]. The presence of cross-reactive Rmp antibodies also facilitates transmission [160] and women with Rmp antibody titers appear at an increased risk for infection [161]. As the Rmp protein is in close association with Por protein [162] it would appear to be imperative that Rmp protein is excluded from any Por-based vaccine preparation. Nonetheless, a quiet optimism now pervades the field that an anti-gonococcal vaccine may be around the corner [151].
MOLECULAR EPIDEMIOLOGY – A HISTORICAL REVIEW
Auxotyping and serotyping – 70’s through the early 80’s
As public health decisions regarding transmissible pathogenic diseases rely heavily on epidemiological surveillance, it became necessary to accurately identify and characterize the different circulating strains of N. gonorrhoeae [163]. Initially, isolates were typed through growth responses on chemically defined media [164][165] or by serotyping using common protein antigens or lipopolysaccharide [41][42][43][166]. Consequently, the identification of different auxotypes allowed different N. gonorrhoeae strains to be typed with respect to disease severity [167][168]. Subsequent Por-based serotyping allowed isolates to initially be grouped into two structurally related forms [41][44][169], which was then further refined using enzyme-linked immunosorbant assays to eventually define nine different Por-based serotypes [170].
–
Attempts were then made to differentiate isolates that caused uncomplicated, localized infections and those that caused disseminated gonococcal infections (DGI) [171]. DGI phenotypes included an increased sensitivity to penicillin [172], unique nutritional requirements [167] coupled with serum resistance which led to increased virulence of DGI isolates [171]. Subsequently, it has been shown that the majority of DGI isolates belonged to two distinct serotypes [43][173].
–
The emergence of antibiotic-resistant strains of N. gonorrhoeae identified a need to determine modes of antibiotic resistance among strains in order to monitor the development of new resistance genes, the lateral transfer of resistance genes, or the spread of resistance strains among the population. Early genetic mapping identified several genes involved in antibiotic resistance [174]. Through epidemiologic studies and characterization of penicillinase-producing N. gonorrhoeae (PPNG), it was determined that two independent strains of PPNG arose in geographically separate populations; both carried the resistance gene on distinct plasmids, with one strain (linked to the Far East) being more prevalent than the strain linked to West Africa [175]. Analysis of PPNG strains demonstrated that their introduction into the United States was due to returning military personnel from the Far East. Travel also contributed to global spread of these strains, as patients would encounter penicillin-resistant β-lactamase-producing N. gonorrhoeae following rendezvous with overseas prostitutes, which would in turn often transmit them to local prostitutes, thereby continuing their spread [168][176].
–
Such analysis of clinical isolates indicated that distinct reservoirs of infection could be detected based upon sexual preference. Studies revealed that homosexual men had a lower incidence of asymptomatic urethral infections and DGIs, yet more frequently acquired infections by strains that were more resistant to penicillin G, which at the time, accounted for the high failure rate of this antibiotic for rectal infections [177]. Also, reservoirs for certain PPNG outbreaks could be traced back to female prostitutes, as these strains were largely absent from the homosexual community. Further epidemiological studies were able to identify gonococci that were exclusively present in both heterosexual men and women, or within homosexual male communities, thus defining sources of infection between male and female partners, prostitution and/or same sex partners [168].
–
“Core group” hypothesis – late 70’s through the 80’s
As previous gonorrheal infections provide little to no immunity to subsequent infections, an alternative model for gonorrhea transmission was proposed, t suggesting that all cases of the disease are caused by a core group of individuals [178]. This “core group” hypothesis, was later reinforced by the emergence and spread of PPNG from the Far East [168][178] and through clinical investigations in the United States [179][180]. The persistence of isolates within a community was proposed to be due to a number of factors including the tendency for these strains to cause asymptomatic infections, or, alternatively, to have long incubation times prior to the onset of symptoms, which provided support to the theory that a core group of transmitters, most likely prostitutes, transmit the disease to many sexual partners [168]. Epidemiological studies revealed that a substantial group of individuals (33%) admitted to continual sexual engagement even with the knowledge of potential exposure, or, worse, even after the onset of symptoms, and that men with new or multiple sex partners were more likely to contract gonorrhea [181][182]. Consequently, five sociological trends were identified that assisted the rise of gonorrhea infections: 1) frequent changes in sex partners, 2) increased population mobility, 3) increasing gonococcal resistance to antibiotics, 4) decreased condom, diaphragm and spermicide use, and 5) increasing the use of oral contraceptives [183].
–
Linkage disequilibrium – 1993
With the widespread use of serological typing, coupled with the desire for vaccine development, the classification and characterization of gonococcal strains invariably focused on investigating surface exposed antigens [184]. However, the combination of auxotyping and serotyping proved to be unreliable, as these techniques did not always provide adequate resolution [185]. As most pathogens are clonal with a disposition towards linkage disequilibrium, this property generally allows for classification based upon nucleotides that are present at variable sites, which in turn allows the serology, pathogenicity, host specificity and the presence of virulence genes to be mapped [184][186]. However, panmictic microorganisms, such as the gonococcus, that undergo mutation and frequent recombinational exchanges, do not allow stable clones to emerge due to the randomization of alleles within a population. Consequently, this complicates epidemiological characterization. Also, as surface-exposed antigens that are used for serotyping also tend to evolve rapidly due to strong diversifying selection placed on them by the host immune system, this further compounds the problem [184]. Given the above problems, it became necessary to index genes that only undergo neutral variation in order to investigate population structure, which led to analysis being focused on housekeeping genes involved in central metabolism [187]. Consequently, novel methods of molecular typing were then devised to define outbreaks based on either local or global epidemiology [163].
–
Multilocus enzyme electrophoresis (MLEE) – 90’s
The advent of multilocus enzyme electrophoresis (MLEE) allowed for the presence or absence of linkage disequilibrium within a population to be monitored via deviations between multiple chromosomal alleles [187][188][189][190]. Indeed, this approach allowed for global epidemiological studies and permitted the identification of strains with an increased tendency to cause disease [163]. Statistical analysis performed on the electrophoretic types of 227 global N. gonorrhoeae isolates provided evidence of a panmictic population structure, as no single pair of alleles was statistically significant for linkage disequilibrium. Additionally, it was determined that the genetic variability of isolates obtained from the same geographic location was as great as that found between all geographic locations that were analyzed. Consequently, it was concluded that the propensity for individual hosts to carry more than one genotype of N. gonorrhoeae, combined with natural competence for DNA transformation, promoted the highly panmictic nature of this pathogen [188].
–
Multilocus sequence typing (MLST) – 90’s
However, MLEE had limitations as it could only detect a small proportion of mutations through differences in electrophoretic mobility [163][184]. Therefore, nucleotide sequencing of the core gene set was then introduced leading to multilocus sequence typing (MLST) [163]. This proved to be extremely effective at detecting relationships between identical or closely related isolates by characterizing them on the basis of sequence variation [163][191]. While MLST typing could be readily applied to N. meningitidis isolates, it was initially thought that clinical isolates of N. gonorrhoeae could not be used, as gonococcal housekeeping genes appeared to be homologous [163][184][192]. Also, as frequent recombination occurred within the organism, it was initially believed that the genetic relatedness of distant isolates may become obscured [193]. However, recombinant exchanges must accrue over long time periods for relationships to be masked, and as the field of molecular epidemiology is only concerned with very short evolutionary time scales, any correlations drawn are unlikely to be skewed by recombination [191]. Therefore, MLST studies did show that N. gonorrhoeae isolates could be typed using the same methods applied to N. meningitidis [163] and N. lactamica [185][194]. It was through comparison of MLST data among the Neisseriae, that it was postulated that minimal interspecies recombination actually occurs among the housekeeping genes [185].
–
eBURST – 2000’s
Typically, MLST allelic profiles were placed into a matrix of pairwise differences which allows for detection of identical or closely related isolates. However, these do not provide the necessary information on the evolutionary descent of genotypic clusters, nor do they identify the founder genotype [191]. Additionally, in bacterial species such as N. gonorrhoeae that undergo frequent recombination, any relatedness that may be implied through the use of pairwise differences is highly suspect and most likely may not be phylogenetically relevant [195]. To account for these concerns, the BURST (based upon related sequence types) algorithm was designed to analyze microbial MLST data by assigning defined sequence types (STs) to lineages which allowed the identification of a putative founder genotype [196].
–
The program was further refined with the development of the eBURST algorithm, which differentiates large MLST datasets based on isolates with the most parsimonious descent pattern from the probable founder, and allows for the identification of clone diversification yet also provides insight into the emergence of clinically relevant isolates [191]. Initially, eBURST was used for analysis of quinolone-resistant N. gonorrhoeae (QRNG) [197]. Previous epidemiological studies of quinolone resistance strains of N. gonorrhoeae could not determine if distinct isolates arose due to variation of an original strain or if multiple strains were concomitantly introduced into a specific geographic location [197][198][199][200]. eBURST analysis determined the total number of QRNG strains that entered a country, the divergence of loci, and the time period during which the founder strains evolved [197]. With the combination of MLST and eBURST analysis, disease isolates could now be defined with regard to distribution, population structure, and evolution [201]. Consequently, the origins of pathogenic strains could now be determined as well as how bacterial populations respond to antibiotics and vaccines through analysis of recent evolutionary changes [202].
CHEMOTHERAPY
Neisseria gonorrhoeae is rapidly evolving and has developed resistance to all previous and current antimicrobials. The recent emergence of multidrug resistant gonococcal isolates in Japan [203], France [204], and Spain [205] has provoked major concerns in public health circles worldwide, especially as drug resistance is spreading rapidly [206]. Consequently, we may be entering an era of untreatable gonorrhea. Medications such as penicillin, and later, the fluoroquinolines, have each been used to treat gonorrhea in the past, however, resistance to these antimicrobial agents quickly developed, leaving limited options for gonococcal treatment [207]. Currently, third generation extended-spectrum cephalosporins (ESCs); which include ceftriaxone (injectable form) and cefixime (oral form) are being prescribed. However, resistance to ESCs has also emerged with resistant isolates having been reported in 17 different countries [208][209].
–
The recent emergence of the first N. gonorrhoeae “superbug” strain in Japan (H041, which was later assigned to MLST ST7363) has been shown to exhibit extremely high-level resistance to all ESCs, including cefixime (MIC= 8 µg/ml), and ceftriaxone (MIC= 2-4 µg/ml) as well as to almost all other available therapeutic antimicrobials [203]. Since the isolation of the H041 strain, other extensive drug resistance (XDR) strains have also been isolated in Quimper, France (F89 strain) [204] as well as in Catalonia, Spain [205], and both share considerable genetic and phenotypic similarity to the Japanese H041 strain. Unfortunately, transmission of these strains is augmented by the fact that XDR strains have been isolated from commercial sex workers, homosexual men, sex tourists, long distance truck drivers, and people undergoing forced migration, suggesting that these strains have the potential to spread globally [206].
–
N. gonorrhoeae are exceptional bacteria that can rapidly evolve to promote adaptation and survival within different niches of the human host. This is facilitated by their natural competence which allows DNA uptake from the environment via transformation, as well as by engaging in bacterial conjugation. Consequently, gonococci can acquire various different types of antimicrobial resistance (AMR), which include drug inactivation, modification of drug targets, changing bacterial permeability barriers, and increasing efflux properties [207][208]. The acquisition of AMR genes was initially thought to occur within commensal Neisseria spp. that reside in the pharynx, as pharyngeal organisms are often exposed to antimicrobials, with the fixed mutations then being transferred to gonococci that mingle with the commensal bacteria [210]. Neisseria can also obtain AMR through spontaneous mutations, although such events are comparatively rare. Many resistance determinants originate through the accumulation of chromosomal mutations, with only two known plasmid-borne genes having been described; penicillin resistance associated with the blaTEM plasmid [211][212][213] and tetracycline resistance associated with the tetM plasmid [214]. Penicillinase-producing strains of Neisseria gonorrhoeae were first isolated in Southeast Asia and in sub-Saharan Africa [175]. However, less than one percent of gonococcal clinical isolates in the US contain the β-lactamase gene, indicating that the major mechanism of penicillin resistance appears to result from accumulation of chromosomal mutations over time [213]. Interestingly, the N. gonorrhoeae tetM conjugative plasmid [215] is not only self-transmissible but is also responsible for transfer of the β-lactamase plasmids to other gonococci, other Neisseria spp., and E. coli [216][217].
–
Chromosomal-mediated resistance to penicillin, as well as to other ESCs, generally involves modification of the penicillin binding proteins (PBP) coupled with mutations that enhance the efflux and decrease the influx of antimicrobials. Penicillin-resistant gonococcal strains typically contain 5 to 9 point mutations in the penA gene which encodes PBP2, the primary lethal target of the β-lactam antimicrobials [218][219]. Penicillin and ESC minimum inhibitory concentrations (MICs) can also be elevated in strains carrying mtrR and penB mutations which increase efflux and decrease influx of the antimicrobials, respectively [203][204]. Surprisingly, synergy between mtrR and penB mutations appears to have very little impact on resistance to cefixime which is mainly conferred by penA mosaic alleles [220].
–
Once acquired, resistance determinants contributing to decreased susceptibility or resistance to certain antibiotics are stably carried within Neisseria populations even when the antibiotic is withdrawn from treatment regimens [207]. The persistence of resistance determinants also suggests that these factors do not cause a negative impact on the biological fitness of the gonococcus. In fact, antibiotic resistance can be linked with enhanced fitness as demonstrated with the MtrCDE efflux system that contributes to gonococcal virulence and survival during an infection [221][222]. This efflux pump can recognize and expel not only hydrophobic antibiotics such as penicillin, ESCs, macrolides, tetracycline, and ciprofloxacin [223][224][225], but also antimicrobial compounds from the innate host response such as antimicrobial peptides, bile salts, and progesterone, allowing the bacteria to survive within host cells [226].
–
Future directions
Due to the lack of an efficacious vaccine, control of gonococcal infections relies on appropriate antibiotic treatment, coupled with prevention, proper diagnosis, and epidemiological surveillance. Recently, novel dual antimicrobial therapy, e.g. ceftriaxone and azithromycin [227][228] or gentamicin and azithromycin [229] combination treatment, has been evaluated for treatment of uncomplicated gonorrhea. However, the emergence of concomitant resistance to the available antimicrobials has again compromised such an approach [206][207][227][230].
–
Previously developed antibiotics, including gentamicin, solithromycin, and ertapenem, are also now being considered as clinical isolates show a high degree of sensitivity to these antibiotics in vitro [231][232]. The carbapenem, ertapenem, is potentially an option for ceftriaxone –resistant N. gonorrhoeae as these strains display relatively low MICs when treated with this agent [233]. However, regimens with ertapenem are only applicable if ertapenem and ceftriaxone do not share the same resistance mechanism such as strains carrying certain penA, mtrR, and penB mutations which coincided with increased carbapenem MICs [208][233]. Consequently, using these antimicrobials may only provide a short-term solution for combating multidrug-resistant gonorrhea [206].
–
To counteract this problem, new antibiotics are being developed for anti-gonococcal therapy. The novel macrolide-family of antibiotics, such as bicyclolides modithromycin (EDP-420) and EDP-322, display high activity against azithromycin-, ESC-, and multidrug-resistant gonococcal isolates in vitro. However, these macrolide drugs appear to cause some cross-resistance with high-level azithromycin resistance [234]. The tetracycline derivatives, glycylcycline tigecycline and fluorocycline eravacycline (TP-434), have also been shown to be effective against ceftriaxone-resistant gonococci in vitro, yet, concerns remain regarding their usage and effectiveness [235][236]. Recently, new broad-spectrum fluoroquinolones, such as avarofloxacin (JNJ-Q2) [237], delafloxacin, sitafloxacin [238], and WQ-3810 [239], have displayed high potency against multidrug-resistant gonococcal isolates in vitro including ciprofloxacin-resistant strains. Finally, the lipoglycopeptide dalbavancin and 2-acyl carbapenems, SM-295291 and SM-369926, are among potential antimicrobials that can be used in gonorrhea treatment to a limited extent [240].
–
Current research has centered on exploring novel antimicrobials or compounds designed to inhibit new targets. Among these newly developed agents are a protein inhibitor (pleuromutilin BC-3781), a boron-containing inhibitor (AN3365) [241], efflux pump inhibitors, which enhance susceptibility to antimicrobials, host innate defense components and toxic metabolites [225][242], non-cytotoxic nanomaterials [243], host defense peptides- LL-37 (multifunctional cathelicidin peptide) [244], molecules that mimic host defensins, LpxC inhibitors [245], species-specific FabI inhibitors (MUT056399) [246], and inhibitors of bacterial topoisomerases (VT12-008911 and AZD0914) both of which target alternative sites other than the fluoroquinolone-binding site [247]. Importantly, all these compounds possess potent in vitro activity against multidrug-resistant gonococcal strains [207][248]. The novel spiropyrimidinetrione antibacterial compound (AZD0914) which inhibits DNA biosynthesis [249] appears to be extremely promising, as no emerging resistance has been observed in diverse multidrug-resistant gonococcal isolates [234]. Consequently, AZD0914 is being seriously considered for its potential use as future oral treatment for gonococcal infections especially as it lacks cross-resistance exhibited by other fluoroquinolone antibiotics [250].
–
Ideally, the future treatment for gonorrhea will rely on individually-tailored regimens as clinical isolates will hopefully be rapidly characterized by novel phenotypic AMR tests and rapid genetic point-of-care antimicrobial resistance tests. Unfortunately, no commercial molecular diagnostic kit is currently available to detect AMR determinants in gonococci, with the current genetic assays lacking sensitivity and specificity [248][251]. Meanwhile, healthcare initiatives need to be immediately undertaken to postpone the further widespread dissemination of ceftriaxone-resistant N. gonorrhoeae strains. These measures should include conducting AMR surveillance on global, national, as well as local scales, identifying treatment failures, monitoring the susceptibility of gonococcal isolates to prescribed antibiotics, and using appropriate and effective antibiotics with optimized quality and doses in gonorrhea treatment regimens [208].
References
- B.E. Britigan, M.S. Cohen, and P.F. Sparling, "Gonococcal Infection: A Model of Molecular Pathogenesis", New England Journal of Medicine, vol. 312, pp. 1683-1694, 1985. http://dx.doi.org/10.1056/NEJM198506273122606
- W.J. MacLennan, B.J. Chapman, M. Smith, R.J. Prescott, and J.X. Wang, "Co-ordinating geriatric and general medical services; experience of a geriatric assessment ward in the Royal Infirmary of Edinburgh.", Scottish medical journal, 1992. http://www.ncbi.nlm.nih.gov/pubmed/1496359
- . World Health Organization, Departement of Reproductive Health and Research, " Global incidence and prevalence of selected curable sexually transmitted infecions - 2008.", World Health Organization., 2012.
- J.N. Wasserheit, "Effect of changes in human ecology and behavior on patterns of sexually transmitted diseases, including human immunodeficiency virus infection.", Proceedings of the National Academy of Sciences, vol. 91, pp. 2430-2435, 1994. http://dx.doi.org/10.1073/pnas.91.7.2430
- J.L. Edwards, and E.K. Butler, "The Pathobiology of Neisseria gonorrhoeae Lower Female Genital Tract Infection", Frontiers in Microbiology, vol. 2, 2011. http://dx.doi.org/10.3389/fmicb.2011.00102
- M.Y. Sutton, M. Sternberg, A. Zaidi, M.E. St. Louis, and L.E. Markowitz, "Trends in Pelvic Inflammatory Disease Hospital Discharges and Ambulatory Visits, United States, 1985–2001", Sexually Transmitted Diseases, vol. 32, pp. 778-784, 2005. http://dx.doi.org/10.1097/01.olq.0000175375.60973.cb
- C.E. French, G. Hughes, A. Nicholson, M. Yung, J.D. Ross, T. Williams, and K. Soldan, "Estimation of the Rate of Pelvic Inflammatory Disease Diagnoses: Trends in England, 2000–2008", Sexually Transmitted Diseases, vol. 38, pp. 158-162, 2011. http://dx.doi.org/10.1097/olq.0b013e3181f22f3e
- J.D.C. Ross, and G. Hughes, "Why is the incidence of pelvic inflammatory disease falling?", BMJ, vol. 348, pp. g1538-g1538, 2014. http://dx.doi.org/10.1136/bmj.g1538
- L. Nathan, A. Decherney, and M. Pernoll, "Current obstetric & gynecologic diagnosis & treatment. 9th edition.", Lange Medical Books/McGraw-Hill, New York, 2003.
- M.S. Cohen, I.F. Hoffman, R.A. Royce, P. Kazembe, J.R. Dyer, C.C. Daly, D. Zimba, P.L. Vernazza, M. Maida, S.A. Fiscus, and J.J. Eron, "Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1", The Lancet, vol. 349, pp. 1868-1873, 1997. http://dx.doi.org/10.1016/S0140-6736(97)02190-9
- C. Hastings, "Public Health Aspects of Venereal Diseases.", Public Health J 8: 37-41., 1917.
- D.S. KELLOGG, W.L. PEACOCK, W.E. DEACON, L. BROWN, and D.I. PIRKLE, "NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION.", Journal of bacteriology, 1963. http://www.ncbi.nlm.nih.gov/pubmed/14047217
- D.S. Kellogg, I.R. Cohen, L.C. Norins, A.L. Schroeter, and G. Reising, "Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro.", Journal of bacteriology, 1968. http://www.ncbi.nlm.nih.gov/pubmed/4979098
- J. Swanson, S.J. Kraus, and E.C. Gotschlich, "STUDIES ON GONOCOCCUS INFECTION", The Journal of Experimental Medicine, vol. 134, pp. 886-906, 1971. http://dx.doi.org/10.1084/jem.134.4.886
- J. Swanson, K. Robbins, O. Barrera, D. Corwin, J. Boslego, J. Ciak, M. Blake, and J.M. Koomey, "Gonococcal pilin variants in experimental gonorrhea.", The Journal of experimental medicine, vol. 165, pp. 1344-1357, 1987. http://dx.doi.org/10.1084/jem.165.5.1344
- W.A. Pearce, and T.M. Buchanan, "Attachment role of gonococcal pili. Optimum conditions and quantitation of adherence of isolated pili to human cells in vitro.", Journal of Clinical Investigation, vol. 61, pp. 931-943, 1978. http://dx.doi.org/10.1172/JCI109018
- D.L. Draper, J.F. James, G.F. Brooks, and R.L. Sweet, "Comparison of virulence markers of peritoneal and fallopian tube isolates with endocervical Neisseria gonorrhoeae isolates from women with acute salpingitis.", Infection and immunity, 1980. http://www.ncbi.nlm.nih.gov/pubmed/6769811
- E.C. Tramont, J. Ciak, J. Boslego, D.G. McChesney, C.C. Brinton, and W. Zollinger, "Antigenic Specificity of Antibodies in Vaginal Secretions during Infection with Neisseria gonorrhoeae", Journal of Infectious Diseases, vol. 142, pp. 23-31, 1980. http://dx.doi.org/10.1093/infdis/142.1.23
- P. Densen, and G.L. Mandell, "Gonococcal interactions with polymorphonuclear neutrophils: importance of the phagosome for bactericidal activity.", Journal of Clinical Investigation, vol. 62, pp. 1161-1171, 1978. http://dx.doi.org/10.1172/JCI109235
- M. Virji, and J.E. Heckels, "The Effect of Protein II and Pili on the Interaction of Neisseria gonorrhoeae with Human Polymorphonuclear Leucocytes", Microbiology, vol. 132, pp. 503-512, 1986. http://dx.doi.org/10.1099/00221287-132-2-503
- J. BOSLEGO, E. TRAMONT, R. CHUNG, D. MCCHESNEY, J. CIAK, J. SADOFF, M. PIZIAK, J. BROWN, C. BRINTONJR, and S. WOOD, "Efficacy trial of a parenteral gonococcal pilus vaccine in men", Vaccine, vol. 9, pp. 154-162, 1991. http://dx.doi.org/10.1016/0264-410X(91)90147-X
- N.E. Freitag, H.S. Seifert, and M. Koomey, "Characterization of the pilF—pilD pilus‐assembly locus of Neisseria gonorrhoeae", Molecular Microbiology, vol. 16, pp. 575-586, 1995. http://dx.doi.org/10.1111/j.1365-2958.1995.tb02420.x
- K.T. Forest, and J.A. Tainer, "Type-4 pilus-structure: outside to inside and top to bottom – aminireview", Gene, vol. 192, pp. 165-169, 1997. http://dx.doi.org/10.1016/S0378-1119(97)00008-5
- P. Hagblom, E. Segal, E. Billyard, and M. So, "Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae", Nature, vol. 315, pp. 156-158, 1985. http://dx.doi.org/10.1038/315156a0
- T. Meyer, and S. Hill, "Genetic variation in the pathogenic Neisseria spp. In: Craig A, Scherf A, editors. Antigenic variation ", Academic Press, pp.142-164., 2003.
- S.A. Hill, and J.K. Davies, "Pilin gene variation inNeisseria gonorrhoeae: reassessing the old paradigms", FEMS Microbiology Reviews, vol. 33, pp. 521-530, 2009. http://dx.doi.org/10.1111/j.1574-6976.2009.00171.x
- A.B. Jonsson, G. Nyberg, and S. Normark, "Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly.", The EMBO journal, 1991. http://www.ncbi.nlm.nih.gov/pubmed/1671354
- T. Tønjum, and M. Koomey, "The pilus colonization factor of pathogenic neisserial species: organelle biogenesis and structure/function relationships – areview", Gene, vol. 192, pp. 155-163, 1997. http://dx.doi.org/10.1016/S0378-1119(97)00018-8
- M. Fussenegger, T. Rudel, R. Barten, R. Ryll, and T.F. Meyer, "Transformation competence and type-4 pilus biogenesis in Neisseriagonorrhoeae – areview", Gene, vol. 192, pp. 125-134, 1997. http://dx.doi.org/10.1016/S0378-1119(97)00038-3
- M. Wolfgang, "Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili", The EMBO Journal, vol. 19, pp. 6408-6418, 2000. http://dx.doi.org/10.1093/emboj/19.23.6408
- S.L. Drake, and M. Koomey, "The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae", Molecular Microbiology, vol. 18, pp. 975-986, 1995. http://dx.doi.org/10.1111/j.1365-2958.1995.18050975.x
- T. Rudel, H. Boxberger, and T.F. Meyer, "Pilus biogenesis and epithelial cell adherence of Neisseria gonorrhoeae pilC double knock‐out mutants", Molecular Microbiology, vol. 17, pp. 1057-1071, 1995. http://dx.doi.org/10.1111/j.1365-2958.1995.mmi_17061057.x
- J.E. Heckels, "Structure and function of pili of pathogenic Neisseria species.", Clinical microbiology reviews, 1989. http://www.ncbi.nlm.nih.gov/pubmed/2566375
- S.D. Goodman, and J.J. Scocca, "Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae.", Proceedings of the National Academy of Sciences, vol. 85, pp. 6982-6986, 1988. http://dx.doi.org/10.1073/pnas.85.18.6982
- M. Koomey, E.C. Gotschlich, K. Robbins, S. Bergström, and J. Swanson, "Effects of recA mutations on pilus antigenic variation and phase transitions in Neisseria gonorrhoeae.", Genetics, 1987. http://www.ncbi.nlm.nih.gov/pubmed/2891588
- M. Wolfgang, J.P.M. Van Putten, S.F. Hayes, and M. Koomey, "The comP locus of Neisseria gonorrhoeae encodes a type IV prepilin that is dispensable for pilus biogenesis but essential for natural transformation", Molecular Microbiology, vol. 31, pp. 1345-1357, 1999. http://dx.doi.org/10.1046/j.1365-2958.1999.01269.x
- F.E. Aas, M. Wolfgang, S. Frye, S. Dunham, C. Løvold, and M. Koomey, "Competence for natural transformation in Neisseria gonorrhoeae: components of DNA binding and uptake linked to type IV pilus expression", Molecular Microbiology, vol. 46, pp. 749-760, 2002. http://dx.doi.org/10.1046/j.1365-2958.2002.03193.x
- A. Cehovin, P.J. Simpson, M.A. McDowell, D.R. Brown, R. Noschese, M. Pallett, J. Brady, G.S. Baldwin, S.M. Lea, S.J. Matthews, and V. Pelicic, "Specific DNA recognition mediated by a type IV pilin", Proceedings of the National Academy of Sciences, vol. 110, pp. 3065-3070, 2013. http://dx.doi.org/10.1073/pnas.1218832110
- M. Wolfgang, P. Lauer, H. Park, L. Brossay, J. Hébert, and M. Koomey, "PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae", Molecular Microbiology, vol. 29, pp. 321-330, 1998. http://dx.doi.org/10.1046/j.1365-2958.1998.00935.x
- B. Maier, M. Koomey, and M.P. Sheetz, "A force-dependent switch reverses type IV pilus retraction", Proceedings of the National Academy of Sciences, vol. 101, pp. 10961-10966, 2004. http://dx.doi.org/10.1073/pnas.0402305101
- K.H. Johnston, K.K. Holmes, and E.C. Gotschlich, "The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity.", The Journal of experimental medicine, vol. 143, pp. 741-758, 1976. http://dx.doi.org/10.1084/jem.143.4.741
- E.G. Sandstrom, K.C. Chen, and T.M. Buchanan, "Serology of Neisseria gonorrhoeae: coagglutination serogroups WI and WII/III correspond to different outer membrane protein I molecules.", Infection and immunity, 1982. http://www.ncbi.nlm.nih.gov/pubmed/6183215
- J.G. Cannon, T.M. Buchanan, and P.F. Sparling, "Confirmation of association of protein I serotype of Neisseria gonorrhoeae with ability to cause disseminated infection.", Infection and immunity, 1983. http://www.ncbi.nlm.nih.gov/pubmed/6404835
- R.C. Judd, "Protein I: structure, function, and genetics.", Clinical microbiology reviews, 1989. http://www.ncbi.nlm.nih.gov/pubmed/2497962
- E. Lynch, M. Blake, E. Gotschlich, and A. Mauro, "Studies of Porins", Biophysical Journal, vol. 45, pp. 104-107, 1984. http://dx.doi.org/10.1016/S0006-3495(84)84127-2
- J.F. Weel, C.T. Hopman, and J.P. van Putten, "Bacterial entry and intracellular processing of Neisseria gonorrhoeae in epithelial cells: immunomorphological evidence for alterations in the major outer membrane protein P.IB.", The Journal of experimental medicine, vol. 174, pp. 705-715, 1991. http://dx.doi.org/10.1084/jem.174.3.705
- A. Muller, "VDAC and the bacterial porin PorB of Neisseria gonorrhoeae share mitochondrial import pathways", The EMBO Journal, vol. 21, pp. 1916-1929, 2002. http://dx.doi.org/10.1093/emboj/21.8.1916
- A. Muller, "Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases", The EMBO Journal, vol. 18, pp. 339-352, 1999. http://dx.doi.org/10.1093/emboj/18.2.339
- P. Massari, Y. Ho, and L.M. Wetzler, "Neisseria meningitidisporin PorB interacts with mitochondria and protects cells from apoptosis", Proceedings of the National Academy of Sciences, vol. 97, pp. 9070-9075, 2000. http://dx.doi.org/10.1073/pnas.97.16.9070
- T. Rudel, A. Schmid, R. Benz, H. Kolb, F. Lang, and T.F. Meyer, "Modulation of Neisseria Porin (PorB) by Cytosolic ATP/GTP of Target Cells: Parallels between Pathogen Accommodation and Mitochondrial Endosymbiosis", Cell, vol. 85, pp. 391-402, 1996. http://dx.doi.org/10.1016/S0092-8674(00)81117-4
- I.M. Mosleh, L.A. Huber, P. Steinlein, C. Pasquali, D. Günther, and T.F. Meyer, "Neisseria gonorrhoeae Porin Modulates Phagosome Maturation", Journal of Biological Chemistry, vol. 273, pp. 35332-35338, 1998. http://dx.doi.org/10.1074/jbc.273.52.35332
- J. Swanson, "Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci.", Infection and immunity, 1978. http://www.ncbi.nlm.nih.gov/pubmed/415006
- J. Swanson, "Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae.", Infection and immunity, 1978. http://www.ncbi.nlm.nih.gov/pubmed/101459
- J. Swanson, "Colony opacity and protein II compositions of gonococci.", Infection and immunity, 1982. http://www.ncbi.nlm.nih.gov/pubmed/6809633
- K.S. Bhat, C.P. Gibbs, O. Barrera, S.G. Morrison, F. Jähnig, A. Stern, E. Kupsch, T.F. Meyer, and J. Swanson, "The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes", Molecular Microbiology, vol. 5, pp. 1889-1901, 1991. http://dx.doi.org/10.1111/j.1365-2958.1991.tb00813.x
- C. Hauck, "'Small' talk: Opa proteins as mediators of Neisseria–host-cell communication", Current Opinion in Microbiology, vol. 6, pp. 43-49, 2003. http://dx.doi.org/10.1016/S1369-5274(03)00004-3
- A. Stern, M. Brown, P. Nickel, and T.F. Meyer, "Opacity genes in Neisseria gonorrhoeae: Control of phase and antigenic variation", Cell, vol. 47, pp. 61-71, 1986. http://dx.doi.org/10.1016/0092-8674(86)90366-1
- J. Swanson, R.J. Belland, and S.A. Hill, "Neisserial surface variation: how and why?", Current Opinion in Genetics & Development, vol. 2, pp. 805-811, 1992. http://dx.doi.org/10.1016/S0959-437X(05)80143-1
- J. Swanson, O. Barrera, J. Sola, and J. Boslego, "Expression of outer membrane protein II by gonococci in experimental gonorrhea.", The Journal of experimental medicine, vol. 168, pp. 2121-2129, 1988. http://dx.doi.org/10.1084/jem.168.6.2121
- A.E. Jerse, M.S. Cohen, P.M. Drown, L.G. Whicker, S.F. Isbey, H.S. Seifert, and J.G. Cannon, "Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male.", The Journal of experimental medicine, vol. 179, pp. 911-920, 1994. http://dx.doi.org/10.1084/jem.179.3.911
- S. Makino, J.P. van Putten, and T.F. Meyer, "Phase variation of the opacity outer membrane protein controls invasion by Neisseria gonorrhoeae into human epithelial cells.", The EMBO journal, 1991. http://www.ncbi.nlm.nih.gov/pubmed/1673923
- J.P. van Putten, and S.M. Paul, "Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeae entry into human mucosal cells.", The EMBO journal, 1995. http://www.ncbi.nlm.nih.gov/pubmed/7774572
- T. Chen, R.J. Belland, J. Wilson, and J. Swanson, "Adherence of pilus- Opa+ gonococci to epithelial cells in vitro involves heparan sulfate.", The Journal of experimental medicine, vol. 182, pp. 511-517, 1995. http://dx.doi.org/10.1084/jem.182.2.511
- T. Chen, and E. Gotschlich, "CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins", Proceedings of the National Academy of Sciences, vol. 93, pp. 14851-14856, 1996. http://dx.doi.org/10.1073/pnas.93.25.14851
- M. Virji, K. Makepeace, D.J.P. Ferguson, and S.M. Watt, "Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae", Molecular Microbiology, vol. 22, pp. 941-950, 1996. http://dx.doi.org/10.1046/j.1365-2958.1996.01551.x
- T. Chen, F. Grunert, A. Medina-Marino, and E.C. Gotschlich, "Several Carcinoembryonic Antigens (CD66) Serve as Receptors for Gonococcal Opacity Proteins", The Journal of Experimental Medicine, vol. 185, pp. 1557-1564, 1997. http://dx.doi.org/10.1084/jem.185.9.1557
- S.D. Gray-Owen, "CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes", The EMBO Journal, vol. 16, pp. 3435-3445, 1997. http://dx.doi.org/10.1093/emboj/16.12.3435
- O. Billker, "Distinct mechanisms of internalization of Neisseria gonorrhoeae by members of the CEACAM receptor family involving Rac1- and Cdc42-dependent and -independent pathways", The EMBO Journal, vol. 21, pp. 560-571, 2002. http://dx.doi.org/10.1093/emboj/21.4.560
- P.A. Rice, and D.L. Kasper, "Characterization of Gonococcal Antigens Responsible for Induction of Bactericidal Antibody in Disseminated Infection", Journal of Clinical Investigation, vol. 60, pp. 1149-1158, 1977. http://dx.doi.org/10.1172/JCI108867
- E.W. Hook, D.A. Olsen, and T.M. Buchanan, "Analysis of the antigen specificity of the human serum immunoglobulin G immune response to complicated gonococcal infection.", Infection and immunity, 1984. http://www.ncbi.nlm.nih.gov/pubmed/6198284
- M.A. Apicella, M.A.J. Westerink, S.A. Morse, H. Schneider, P.A. Rice, and J.M. Griffiss, "Bactericidal Antibody Response of Normal Human Serum to the Lipooligosaccharide of Neisseria gonorrhoeae", Journal of Infectious Diseases, vol. 153, pp. 520-526, 1986. http://dx.doi.org/10.1093/infdis/153.3.520
- C.R. Gregg, M.A. Melly, C.G. Hellerqvist, J.G. Coniglio, and Z.A. McGee, "Toxic Activity of Purified Lipopolysaccharide of Neisseria gonorrhoeae for Human Fallopian Tube Mucosa", Journal of Infectious Diseases, vol. 143, pp. 432-439, 1981. http://dx.doi.org/10.1093/infdis/143.3.432
- H. Schneider, J.M. Griffiss, J.W. Boslego, P.J. Hitchcock, K.M. Zahos, and M.A. Apicella, "Expression of paragloboside-like lipooligosaccharides may be a necessary component of gonococcal pathogenesis in men.", The Journal of experimental medicine, vol. 174, pp. 1601-1605, 1991. http://dx.doi.org/10.1084/jem.174.6.1601
- J.P.M. van Putten, and B.D. Robertson, "Molecular mechanisms and implications for infection of lipopolysaccharide variation in Neisseria", Molecular Microbiology, vol. 16, pp. 847-853, 1995. http://dx.doi.org/10.1111/j.1365-2958.1995.tb02312.x
- R.J. Danaher, J.C. Levin, D. Arking, C.L. Burch, R. Sandlin, and D.C. Stein, "Genetic basis of Neisseria gonorrhoeae lipooligosaccharide antigenic variation.", Journal of bacteriology, 1995. http://www.ncbi.nlm.nih.gov/pubmed/8522539
- Q.L. Yang, and E.C. Gotschlich, "Variation of gonococcal lipooligosaccharide structure is due to alterations in poly-G tracts in lgt genes encoding glycosyl transferases.", The Journal of experimental medicine, vol. 183, pp. 323-327, 1996. http://dx.doi.org/10.1084/jem.183.1.323
- F.P. Winstanley, C.C. Blackwell, E.L. Tan, P.V. Patel, N.J. Parsons, P.M.V. Martin, and H. Smith, "Alteration of Pyocin-sensitivity Pattern of Neisseria gonorrhoeae is Associated with Induced Resistance to Killing by Human Serum", Microbiology, vol. 130, pp. 1303-1306, 1984. http://dx.doi.org/10.1099/00221287-130-5-1303
- N.J. Parsons, J.R. Andrade, P.V. Patel, J.A. Cole, and H. Smith, "Sialylation of lipopolysaccharide and loss of absorption of bactericidal antibody during conversion of gonococci to serum resistance by cytidine 5′-monophospho-N-acetyl neuraminic acid", Microbial Pathogenesis, vol. 7, pp. 63-72, 1989. http://dx.doi.org/10.1016/0882-4010(89)90112-5
- R.E. Mandrell, J. Griffiss, H. Smith, and J.A. Cole, "Distribution of a lipooligosaccharide-specific sialyltransferase in pathogenic and non-pathogenic Neisseria", Microbial Pathogenesis, vol. 14, pp. 315-327, 1993. http://dx.doi.org/10.1006/mpat.1993.1031
- H.D.L. Paz, S.J. Cooke, and J.E. Heckels, "Effect of sialylation of lipopolysaccharide of Neisseria gonorrhoeae on recognition and complement-mediated killing by monoclonal antibodies directed against different outer-membrane antigens", Microbiology, vol. 141, pp. 913-920, 1995. http://dx.doi.org/10.1099/13500872-141-4-913
- C. Elkins, N.H. Carbonetti, V.A. Varela, D. Stirewalt, D.G. Klapper, and P.F. Sparling, "Antibodies to N‐terminal peptides of gonococcal porin are bactericidal when gonococcal lipopolysaccharide is not sialylated", Molecular Microbiology, vol. 6, pp. 2617-2628, 1992. http://dx.doi.org/10.1111/j.1365-2958.1992.tb01439.x
- J.P. van Putten, "Phase variation of lipopolysaccharide directs interconversion of invasive and immuno-resistant phenotypes of Neisseria gonorrhoeae.", The EMBO journal, 1993. http://www.ncbi.nlm.nih.gov/pubmed/7693451
- R. Halter, J. Pohlner, and T.F. Meyer, "Mosaic-like organization of IgA protease genes in Neisseria gonorrhoeae generated by horizontal genetic exchange in vivo.", The EMBO journal, 1989. http://www.ncbi.nlm.nih.gov/pubmed/2511009
- J. Pohlner, R. Halter, K. Beyreuther, and T.F. Meyer, "Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease", Nature, vol. 325, pp. 458-462, 1987. http://dx.doi.org/10.1038/325458a0
- R. Halter, J. Pohlner, and T.F. Meyer, "IgA protease of Neisseria gonorrhoeae: isolation and characterization of the gene and its extracellular product.", The EMBO journal, 1984. http://www.ncbi.nlm.nih.gov/pubmed/6430698
- P. Ayala, B. Vasquez, L. Wetzler, and M. So, "Neisseria gonorrhoeae Porin P1.B Induces Endosome Exocytosis and a Redistribution of Lamp1 to the Plasma Membrane", Infection and Immunity, vol. 70, pp. 5965-5971, 2002. http://dx.doi.org/10.1128/IAI.70.11.5965-5971.2002
- S. Hopper, B. Vasquez, A. Merz, S. Clary, J.S. Wilbur, and M. So, "Effects of the Immunoglobulin A1 Protease on Neisseria gonorrhoeae Trafficking across Polarized T84 Epithelial Monolayers", Infection and Immunity, vol. 68, pp. 906-911, 2000. http://dx.doi.org/10.1128/IAI.68.2.906-911.2000
- C.N. Cornelissen, "Molecular Pathogenesis of Neisseria Gonorrhoeae", Frontiers in Microbiology, vol. 2, 2011. http://dx.doi.org/10.3389/fmicb.2011.00224
- X. Nassif, C. Pujol, P. Morand, and E. Eugène, "Interactions of pathogenic Neisseria with host cells. Is it possible to assemble the puzzle?", Molecular Microbiology, vol. 32, pp. 1124-1132, 1999. http://dx.doi.org/10.1046/j.1365-2958.1999.01416.x
- J.M. Griffiss, C.J. Lammel, J. Wang, N.P. Dekker, and G.F. Brooks, "Neisseria gonorrhoeae coordinately uses Pili and Opa to activate HEC-1-B cell microvilli, which causes engulfment of the gonococci.", Infection and immunity, 1999. http://www.ncbi.nlm.nih.gov/pubmed/10377128
- J.L. Edwards, and M.A. Apicella, "The Molecular Mechanisms Used byNeisseria gonorrhoeaeTo Initiate Infection Differ between Men and Women", Clinical Microbiology Reviews, vol. 17, pp. 965-981, 2004. http://dx.doi.org/10.1128/CMR.17.4.965-981.2004
- H. Källström, M.K. Liszewski, J.P. Atkinson, and A. Jonsson, "Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria", Molecular Microbiology, vol. 25, pp. 639-647, 1997. http://dx.doi.org/10.1046/j.1365-2958.1997.4841857.x
- J. Wang, S.D. Gray‐Owen, A. Knorre, T.F. Meyer, and C. Dehio, "Opa binding to cellular CD66 receptors mediates the transcellular traversal of Neisseria gonorrhoeae across polarized T84 epithelial cell monolayers", Molecular Microbiology, vol. 30, pp. 657-671, 1998. http://dx.doi.org/10.1046/j.1365-2958.1998.01102.x
- J.F. James, and J. Swanson, "Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures.", Infection and immunity, 1978. http://www.ncbi.nlm.nih.gov/pubmed/415007
- K.V. Swanson, G.A. Jarvis, G.F. Brooks, B.J. Barham, M.D. Cooper, and J.M. Griffiss, "CEACAM is not necessary for Neisseria gonorrhoeae to adhere to and invade female genital epithelial cells", Cellular Microbiology, vol. 3, pp. 681-691, 2001. http://dx.doi.org/10.1046/j.1462-5822.2001.00147.x
- A. Sintsova, H. Wong, K.S. MacDonald, R. Kaul, M. Virji, and S.D. Gray-Owen, "Selection for a CEACAM Receptor-Specific Binding Phenotype during Neisseria gonorrhoeae Infection of the Human Genital Tract", Infection and Immunity, vol. 83, pp. 1372-1383, 2015. http://dx.doi.org/10.1128/iai.03123-14
- J.G. Cole, N.B. Fulcher, and A.E. Jerse, "Opacity Proteins Increase Neisseria gonorrhoeae Fitness in the Female Genital Tract Due to a Factor under Ovarian Control", Infection and Immunity, vol. 78, pp. 1629-1641, 2010. http://dx.doi.org/10.1128/IAI.00996-09
- G.J. King, and J. Swanson, "Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association.", Infection and immunity, 1978. http://www.ncbi.nlm.nih.gov/pubmed/211086
- R.F. Rest, S.H. Fischer, Z.Z. Ingham, and J.F. Jones, "Interactions of Neisseria gonorrhoeae with human neutrophils: effects of serum and gonococcal opacity on phagocyte killing and chemiluminescence.", Infection and immunity, 1982. http://www.ncbi.nlm.nih.gov/pubmed/6806195
- R.F. Rest, N. Lee, and C. Bowden, "Stimulation of human leukocytes by protein II+ gonococci is mediated by lectin-like gonococcal components.", Infection and immunity, 1985. http://www.ncbi.nlm.nih.gov/pubmed/3930402
- K.H. Ramsey, H. Schneider, A.S. Cross, J.W. Boslego, D.L. Hoover, T.L. Staley, R.A. Kuschner, and C.D. Deal, "Inflammatory Cytokines Produced in Response to Experimental Human Gonorrhea", Journal of Infectious Diseases, vol. 172, pp. 186-191, 1995. http://dx.doi.org/10.1093/infdis/172.1.186
- P.L. Fisette, S. Ram, J.M. Andersen, W. Guo, and R.R. Ingalls, "The Lip Lipoprotein from Neisseria gonorrhoeae Stimulates Cytokine Release and NF-κB Activation in Epithelial Cells in a Toll-like Receptor 2-dependent Manner", Journal of Biological Chemistry, vol. 278, pp. 46252-46260, 2003. http://dx.doi.org/10.1074/jbc.M306587200
- B.L. Makepeace, P.J. Watt, J.E. Heckels, and M. Christodoulides, "Interactions ofNeisseria gonorrhoeaewith Mature Human Macrophage Opacity Proteins Influence Production of Proinflammatory Cytokines", Infection and Immunity, vol. 69, pp. 1909-1913, 2001. http://dx.doi.org/10.1128/IAI.69.3.1909-1913.2001
- M. Kaparakis, L. Turnbull, L. Carneiro, S. Firth, H.A. Coleman, H.C. Parkington, L. Le Bourhis, A. Karrar, J. Viala, J. Mak, M.L. Hutton, J.K. Davies, P.J. Crack, P.J. Hertzog, D.J. Philpott, S.E. Girardin, C.B. Whitchurch, and R.L. Ferrero, "Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells", Cellular Microbiology, vol. 12, pp. 372-385, 2010. http://dx.doi.org/10.1111/j.1462-5822.2009.01404.x
- N.M. Ovcinnikov, and V.V. Delektorskij, "Electron microscope studies of gonococci in the urethral secretions of patients with gonorrhoea.", Sexually Transmitted Infections, vol. 47, pp. 419-439, 1971. http://dx.doi.org/10.1136/sti.47.6.419
- B. Feinen, A.E. Jerse, S.L. Gaffen, and M.W. Russell, "Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection", Mucosal Immunology, vol. 3, pp. 312-321, 2010. http://dx.doi.org/10.1038/mi.2009.139
- K.L. Seib, M.P. Simons, H. Wu, A.G. McEwan, W.M. Nauseef, M.A. Apicella, and M.P. Jennings, "Investigation of Oxidative Stress Defenses of Neisseria gonorrhoeae by Using a Human Polymorphonuclear Leukocyte Survival Assay", Infection and Immunity, vol. 73, pp. 5269-5272, 2005. http://dx.doi.org/10.1128/IAI.73.8.5269-5272.2005
- A.K. Criss, and H.S. Seifert, "A bacterial siren song: intimate interactions between Neisseria and neutrophils", Nature Reviews Microbiology, vol. 10, pp. 178-190, 2012. http://dx.doi.org/10.1038/nrmicro2713
- K.L. Seib, H. Wu, S.P. Kidd, M.A. Apicella, M.P. Jennings, and A.G. McEwan, "Defenses against Oxidative Stress inNeisseria gonorrhoeae: a System Tailored for a Challenging Environment", Microbiology and Molecular Biology Reviews, vol. 70, pp. 344-361, 2006. http://dx.doi.org/10.1128/MMBR.00044-05
- S. Hill, "Stress responses in pathogenic Neisseria: overlapping regulons and sRNA regulation. In: Kidd S, editor. Stress Response in Pathogenic Bacteria. Advances in Molecular and Cellular Microbiology (Book 19)", Cambridge University Press, pp.115-132., 2011.
- E.A. Stohl, A.K. Criss, and H.S. Seifert, "The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterized roles in oxidative damage protection", Molecular Microbiology, vol. 58, pp. 520-532, 2005. http://dx.doi.org/10.1111/j.1365-2958.2005.04839.x
- C.P. Doherty, "Host-pathogen interactions: the role of iron.", The Journal of nutrition, 2007. http://www.ncbi.nlm.nih.gov/pubmed/17449603
- P.W. Jordan, and N.J. Saunders, "Host Iron Binding Proteins Acting as Niche Indicators for Neisseria meningitidis", PLoS ONE, vol. 4, pp. e5198, 2009. http://dx.doi.org/10.1371/journal.pone.0005198
- E.P. Skaar, "The Battle for Iron between Bacterial Pathogens and Their Vertebrate Hosts", PLoS Pathogens, vol. 6, pp. e1000949, 2010. http://dx.doi.org/10.1371/journal.ppat.1000949
-
N. Noinaj, S.K. Buchanan, and C.N. Cornelissen, "The transferrin–iron import system from pathogenic
N eisseria species", Molecular Microbiology, vol. 86, pp. 246-257, 2012. http://dx.doi.org/10.1111/mmi.12002 - J.M. Noto, and C.N. Cornelissen, "Identification of TbpA Residues Required for Transferrin-Iron Utilization by Neisseria gonorrhoeae", Infection and Immunity, vol. 76, pp. 1960-1969, 2008. http://dx.doi.org/10.1128/IAI.00020-08
- C.N. Cornelissen, G.D. Biswas, J. Tsai, D.K. Paruchuri, S.A. Thompson, and P.F. Sparling, "Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors.", Journal of bacteriology, 1992. http://www.ncbi.nlm.nih.gov/pubmed/1325963
- C.N. Cornelissen, M. Kelley, M.M. Hobbs, J.E. Anderson, J.G. Cannon, M.S. Cohen, and P.F. Sparling, "The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers", Molecular Microbiology, vol. 27, pp. 611-616, 1998. http://dx.doi.org/10.1046/j.1365-2958.1998.00710.x
- D.L. Kasper, P.A. Rice, and W.M. McCormack, "Bactericidal Antibody in Genital Infection Due to Neisseria gonorrhoeae", Journal of Infectious Diseases, vol. 135, pp. 243-251, 1977. http://dx.doi.org/10.1093/infdis/135.2.243
- M.E. WARD, P.R. LAMBDEN, J.E. HECKELS, and P.J. WATT, "The Surface Properties of Neisseria gonorrhoeae: Determinants of Susceptibility to Antibody Complement Killing", Journal of General Microbiology, vol. 108, pp. 205-212, 1978. http://dx.doi.org/10.1099/00221287-108-2-205
- G.K. Schoolnik, T.M. Buchanan, and K.K. Holmes, "Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera.", Journal of Clinical Investigation, vol. 58, pp. 1163-1173, 1976. http://dx.doi.org/10.1172/JCI108569
- K.A. Joiner, K.A. Warren, E.J. Brown, J. Swanson, and M.M. Frank, "Studies on the mechanism of bacterial resistance to complement-mediated killing. IV. C5b-9 forms high molecular weight complexes with bacterial outer membrane constituents on serum-resistant but not on serum-sensitive Neisseria gonorrhoeae.", Journal of immunology (Baltimore, Md. : 1950), 1983. http://www.ncbi.nlm.nih.gov/pubmed/6411816
- K.A. Joiner, K.A. Warren, C. Hammer, and M.M. Frank, "Bactericidal but not nonbactericidal C5b-9 is associated with distinctive outer membrane proteins in Neisseria gonorrhoeae.", Journal of immunology (Baltimore, Md. : 1950), 1985. http://www.ncbi.nlm.nih.gov/pubmed/3918112
- L.A. Lewis, W.M. Shafer, T. Dutta Ray, S. Ram, and P.A. Rice, "Phosphoethanolamine Residues on the Lipid A Moiety of Neisseria gonorrhoeae Lipooligosaccharide Modulate Binding of Complement Inhibitors and Resistance to Complement Killing", Infection and Immunity, vol. 81, pp. 33-42, 2013. http://dx.doi.org/10.1128/IAI.00751-12
- K.A. Joiner, R. Scales, K.A. Warren, M.M. Frank, and P.A. Rice, "Mechanism of action of blocking immunoglobulin G for Neisseria gonorrhoeae.", Journal of Clinical Investigation, vol. 76, pp. 1765-1772, 1985. http://dx.doi.org/10.1172/JCI112167
- G.F. Brooks, C.J. Lammel, B.H. Petersen, and D.P. Stites, "Human seminal plasma inhibition of antibody complement-mediated killing and opsonization of Neisseria gonorrhoeae and other gram-negative organisms.", Journal of Clinical Investigation, vol. 67, pp. 1523-1531, 1981. http://dx.doi.org/10.1172/JCI110183
- S. Ram, D.P. McQuillen, S. Gulati, C. Elkins, M.K. Pangburn, and P.A. Rice, "Binding of Complement Factor H to Loop 5 of Porin Protein 1A: A Molecular Mechanism of Serum Resistance of Nonsialylated Neisseria gonorrhoeae ", The Journal of Experimental Medicine, vol. 188, pp. 671-680, 1998. http://dx.doi.org/10.1084/jem.188.4.671
- J. Ngampasutadol, S. Ram, S. Gulati, S. Agarwal, C. Li, A. Visintin, B. Monks, G. Madico, and P.A. Rice, "Human Factor H Interacts Selectively with Neisseria gonorrhoeae and Results in Species-Specific Complement Evasion", The Journal of Immunology, vol. 180, pp. 3426-3435, 2008. http://dx.doi.org/10.4049/jimmunol.180.5.3426
- J. Ngampasutadol, S. Ram, A.M. Blom, H. Jarva, A.E. Jerse, E. Lien, J. Goguen, S. Gulati, and P.A. Rice, "Human C4b-binding protein selectively interacts withNeisseria gonorrhoeaeand results in species-specific infection", Proceedings of the National Academy of Sciences, vol. 102, pp. 17142-17147, 2005. http://dx.doi.org/10.1073/pnas.0506471102
- A.M. Blom, and S. Ram, "Contribution of interactions between complement inhibitor C4b-binding protein and pathogens to their ability to establish infection with particular emphasis on Neisseria gonorrhoeae", Vaccine, vol. 26, pp. I49-I55, 2008. http://dx.doi.org/10.1016/j.vaccine.2008.11.049
- E.C. Tramont, "Inhibition of adherence of Neisseria gonorrhoeae by human genital secretions.", Journal of Clinical Investigation, vol. 59, pp. 117-124, 1977. http://dx.doi.org/10.1172/JCI108608
- S.R. Hedges, M.S. Mayo, J. Mestecky, E.W. Hook, and M.W. Russell, "Limited local and systemic antibody responses to Neisseria gonorrhoeae during uncomplicated genital infections.", Infection and immunity, 1999. http://www.ncbi.nlm.nih.gov/pubmed/10417159
- I.C. Boulton, and S.D. Gray-Owen, "Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes", Nature Immunology, vol. 3, pp. 229-236, 2002. http://dx.doi.org/10.1038/ni769
- H.S.W. Lee, M.A. Ostrowski, and S.D. Gray-Owen, "CEACAM1 Dynamics during Neisseria gonorrhoeae Suppression of CD4+ T Lymphocyte Activation", The Journal of Immunology, vol. 180, pp. 6827-6835, 2008. http://dx.doi.org/10.4049/jimmunol.180.10.6827
- M. Pantelic, Y. Kim, S. Bolland, I. Chen, J. Shively, and T. Chen, "Neisseria gonorrhoeaeKills Carcinoembryonic Antigen-Related Cellular Adhesion Molecule 1 (CD66a)-Expressing Human B Cells and Inhibits Antibody Production", Infection and Immunity, vol. 73, pp. 4171-4179, 2005. http://dx.doi.org/10.1128/IAI.73.7.4171-4179.2005
- P. Zhang, O. Schwartz, M. Pantelic, G. Li, Q. Knazze, C. Nobile, M. Radovich, J. He, S. Hong, J. Klena, and T. Chen, "DC-SIGN (CD209) recognition of Neisseria gonorrhoeae is circumvented by lipooligosaccharide variation", Journal of Leukocyte Biology, vol. 79, pp. 731-738, 2006. http://dx.doi.org/10.1189/jlb.0405184
- M. Lu, M. Zhang, A. Takashima, J. Weiss, M.A. Apicella, X. Li, D. Yuan, and R.S. Munford, "Lipopolysaccharide deacylation by an endogenous lipase controls innate antibody responses to Gram-negative bacteria", Nature Immunology, vol. 6, pp. 989-994, 2005. http://dx.doi.org/10.1038/ni1246
- A.E. Jerse, "Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice.", Infection and immunity, 1999. http://www.ncbi.nlm.nih.gov/pubmed/10531218
- A.E. Jerse, H. Wu, M. Packiam, R.A. Vonck, A.A. Begum, and L.E. Garvin, "Estradiol-Treated Female Mice as Surrogate Hosts for Neisseria gonorrhoeae Genital Tract Infections", Frontiers in Microbiology, vol. 2, 2011. http://dx.doi.org/10.3389/fmicb.2011.00107
- Y. Liu, and M.W. Russell, "Diversion of the Immune Response to Neisseria gonorrhoeae from Th17 to Th1/Th2 by Treatment with Anti-Transforming Growth Factor β Antibody Generates Immunological Memory and Protective Immunity", mBio, vol. 2, 2011. http://dx.doi.org/10.1128/mBio.00095-11
- Y. Liu, E.A. Islam, G.A. Jarvis, S.D. Gray-Owen, and M.W. Russell, "Neisseria gonorrhoeae selectively suppresses the development of Th1 and Th2 cells, and enhances Th17 cell responses, through TGF-β-dependent mechanisms", Mucosal Immunology, vol. 5, pp. 320-331, 2012. http://dx.doi.org/10.1038/mi.2012.12
- N.S.Y. So, M.A. Ostrowski, and S.D. Gray-Owen, "Vigorous Response of Human Innate Functioning IgM Memory B Cells upon Infection by Neisseria gonorrhoeae", The Journal of Immunology, vol. 188, pp. 4008-4022, 2012. http://dx.doi.org/10.4049/jimmunol.1100718
- P. Muenzner, V. Bachmann, W. Zimmermann, J. Hentschel, and C.R. Hauck, "Human-Restricted Bacterial Pathogens Block Shedding of Epithelial Cells by Stimulating Integrin Activation", Science, vol. 329, pp. 1197-1201, 2010. http://dx.doi.org/10.1126/science.1190892
- G. Li, H. Jiao, H. Yan, J. Wang, X. Wang, and M. Ji, "Establishment of a human CEACAM1 transgenic mouse model for the study of gonococcal infections", Journal of Microbiological Methods, vol. 87, pp. 350-354, 2011. http://dx.doi.org/10.1016/j.mimet.2011.09.012
- L.A. Snyder, S.A. Butcher, and N.J. Saunders, "Comparative whole-genome analyses reveal over 100 putative phase-variable genes in the pathogenic Neisseria spp.", Microbiology, vol. 147, pp. 2321-2332, 2001. http://dx.doi.org/10.1099/00221287-147-8-2321
- R. Moxon, C. Bayliss, and D. Hood, "Bacterial Contingency Loci: The Role of Simple Sequence DNA Repeats in Bacterial Adaptation", Annual Review of Genetics, vol. 40, pp. 307-333, 2006. http://dx.doi.org/10.1146/annurev.genet.40.110405.090442
- R. Haas, S. Veit, and T. Meyer, "Silent pilin genes of Neisseria gonorrhoeae MS11 and the occurrence of related hypervariant sequences among other gonococcal isolates", Molecular Microbiology, vol. 6, pp. 197-208, 1992. http://dx.doi.org/10.1111/j.1365-2958.1992.tb02001.x
- M.T. Anderson, L. Dewenter, B. Maier, and H.S. Seifert, "Seminal Plasma Initiates a Neisseria gonorrhoeae Transmission State", mBio, vol. 5, 2014. http://dx.doi.org/10.1128/mBio.01004-13
- P.F. Sparling, C. Elkins, P.B. Wyrick, and M.S. Cohen, "Vaccines for bacterial sexually transmitted infections: a realistic goal?", Proceedings of the National Academy of Sciences, vol. 91, pp. 2456-2463, 1994. http://dx.doi.org/10.1073/pnas.91.7.2456
- M.W. Russell, S.R. Hedges, H. Wu, E.W. Hook, and J. Mestecky, "Mucosal Immunity in the Genital Tract: Prospects for Vaccines Against Sexually Transmitted Diseases—A Review", American Journal of Reproductive Immunology, vol. 42, pp. 58-63, 1999. http://dx.doi.org/10.1111/j.1600-0897.1999.tb00466.x
- A.E. Jerse, M.C. Bash, and M.W. Russell, "Vaccines against gonorrhea: Current status and future challenges", Vaccine, vol. 32, pp. 1579-1587, 2014. http://dx.doi.org/10.1016/j.vaccine.2013.08.067
- D. McChesney, E.C. Tramont, J.W. Boslego, J. Ciak, J. Sadoff, and C.C. Brinton, "Genital antibody response to a parenteral gonococcal pilus vaccine.", Infection and immunity, 1982. http://www.ncbi.nlm.nih.gov/pubmed/6124502
- E.C. Tramont, and J.W. Boslego, "Pilus vaccines", Vaccine, vol. 3, pp. 3-10, 1985. http://dx.doi.org/10.1016/0264-410X(85)90003-9
- S. Simpson, Y. Ho, P. Rice, and L. Wetzler, "T Lymphocyte Response toNeisseria gonorrhoeaePorin in Individuals with Mucosal Gonococcal Infections", The Journal of Infectious Diseases, vol. 180, pp. 762-773, 1999. http://dx.doi.org/10.1086/314969
- P. Massari, S. Ram, H. Macleod, and L.M. Wetzler, "The role of porins in neisserial pathogenesis and immunity", Trends in Microbiology, vol. 11, pp. 87-93, 2003. http://dx.doi.org/10.1016/S0966-842X(02)00037-9
- T.E. Singleton, P. Massari, and L.M. Wetzler, "Neisserial Porin-Induced Dendritic Cell Activation Is MyD88 and TLR2 Dependent", The Journal of Immunology, vol. 174, pp. 3545-3550, 2005. http://dx.doi.org/10.4049/jimmunol.174.6.3545
- W. Zhu, C.E. Thomas, C. Chen, C.N. Van Dam, R.E. Johnston, N.L. Davis, and P.F. Sparling, "Comparison of Immune Responses to Gonococcal PorB Delivered as Outer Membrane Vesicles, Recombinant Protein, or Venezuelan Equine Encephalitis Virus Replicon Particles", Infection and Immunity, vol. 73, pp. 7558-7568, 2005. http://dx.doi.org/10.1128/IAI.73.11.7558-7568.2005
- Y. Liang, H. Hasturk, J. Elliot, A. Noronha, X. Liu, L.M. Wetzler, P. Massari, A. Kantarci, H.S. Winter, and F.A. Farraye, "Toll-like receptor 2 induces mucosal homing receptor expression and IgA production by human B cells", Clinical Immunology, vol. 138, pp. 33-40, 2011. http://dx.doi.org/10.1016/j.clim.2010.09.003
- P.A. Rice, and D.L. Kasper, "Characterization of Serum Resistance of Neisseria gonorrhoeae that Disseminate", Journal of Clinical Investigation, vol. 70, pp. 157-167, 1982. http://dx.doi.org/10.1172/jci110589
- P.A. RICE, D.P. McQUILLEN, S. GULATI, D.B. JANI, L.M. WETZLER, M.S. BLAKE, and E.C. GOTSCHLICH, "Serum Resistance of Neisseria gonorrhoeae Does It Thwart the Inflammatory Response and Facilitate the Transmission of Infection?a", Annals of the New York Academy of Sciences, vol. 730, pp. 7-14, 1994. http://dx.doi.org/10.1111/j.1749-6632.1994.tb44234.x
- F.A. Plummer, H. Chubb, J.N. Simonsen, M. Bosire, L. Slaney, I. Maclean, J.O. Ndinya-Achola, P. Waiyaki, and R.C. Brunham, "Antibody to Rmp (outer membrane protein 3) increases susceptibility to gonococcal infection.", Journal of Clinical Investigation, vol. 91, pp. 339-343, 1993. http://dx.doi.org/10.1172/jci116190
- W.J. Newhall, W.D. Sawyer, and R.A. Haak, "Cross-linking analysis of the outer membrane proteins of Neisseria gonorrhoeae.", Infection and immunity, 1980. http://www.ncbi.nlm.nih.gov/pubmed/6772567
- M.C.J. Maiden, J.A. Bygraves, E. Feil, G. Morelli, J.E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D.A. Caugant, I.M. Feavers, M. Achtman, and B.G. Spratt, "Multilocus sequence typing: A portable approach to the identification of clones within populations of pathogenic microorganisms", Proceedings of the National Academy of Sciences, vol. 95, pp. 3140-3145, 1998. http://dx.doi.org/10.1073/pnas.95.6.3140
- K. Carifo, and B.W. Catlin, "Neisseria gonorrhoeae auxotyping: differentiation of clinical isolates based on growth responses on chemically defined media.", Applied microbiology, 1973. http://www.ncbi.nlm.nih.gov/pubmed/4201637
- L.J. La Scolea, and F.E. Young, "Development of a defined minimal medium for the growth of Neisseria gonorrhoeae.", Applied microbiology, 1974. http://www.ncbi.nlm.nih.gov/pubmed/4210728
- M.A. Apicella, M. Shero, G.A. Jarvis, J.M. Griffiss, R.E. Mandrell, and H. Schneider, "Phenotypic variation in epitope expression of the Neisseria gonorrhoeae lipooligosaccharide.", Infection and immunity, 1987. http://www.ncbi.nlm.nih.gov/pubmed/2440807
- J.S. Knapp, and K.K. Holmes, "Disseminated Gonococcal Infections Caused by Neisseria gonorrhoeae with Unique Nutritional Requirements", Journal of Infectious Diseases, vol. 132, pp. 204-208, 1975. http://dx.doi.org/10.1093/infdis/132.2.204
- J.S. KNAPP, K.K. HOLMES, P. BONIN, and E.W. HOOK, "Epidemiology of Gonorrhea", Sexually Transmitted Diseases, vol. 14, pp. 26-32, 1987. http://dx.doi.org/10.1097/00007435-198701000-00006
- J.S. Knapp, M.R. Tam, R.C. Nowinski, K.K. Holmes, and E.G. Sandstrom, "Serological Classification of Neisseria gonorrhoeae with Use of Monoclonal Antibodies to Gonococcal Outer Membrane Protein I", Journal of Infectious Diseases, vol. 150, pp. 44-48, 1984. http://dx.doi.org/10.1093/infdis/150.1.44
- T.M. Buchanan, and J.F. Hildebrandt, "Antigen-specific serotyping of Neisseria gonorrhoeae: characterization based upon principal outer membrane protein.", Infection and immunity, 1981. http://www.ncbi.nlm.nih.gov/pubmed/6166568
- B.I. Eisenstein, T.J. Lee, and P.F. Sparling, "Penicillin sensitivity and serum resistance are independent attributes of strains of Neisseria gonorrhoeae causing disseminated gonococcal infection.", Infection and immunity, 1977. http://www.ncbi.nlm.nih.gov/pubmed/404247
- P.J. Wiesner, H.H. Handsfield, and K.K. Holmes, "Low Antibiotic Resistance of Gonococci Causing Disseminated Infection", New England Journal of Medicine, vol. 288, pp. 1221-1222, 1973. http://dx.doi.org/10.1056/NEJM197306072882308
- J.P. OʼBRIEN, D.L. GOLDENBERG, and P.A. RICE, "Disseminated Gonococcal Infection", Medicine, vol. 62, pp. 395, 1983. http://dx.doi.org/10.1097/00005792-198311000-00005
- F.A. Sarubbi, E. Blackman, and P.F. Sparling, "Genetic mapping of linked antibiotic resistance loci in Neisseria gonorrhoeae.", Journal of bacteriology, 1974. http://www.ncbi.nlm.nih.gov/pubmed/4279905
- P. Perine, W. Schalla, M. Siegel, C. Thornsberry, J. Biddle, K. Wong, and S. Thompson, "EVIDENCE FOR TWO DISTINCT TYPES OF PENICILLINASE-PRODUCING NEISSERIA GONORRHή", The Lancet, vol. 310, pp. 993-995, 1977. http://dx.doi.org/10.1016/S0140-6736(77)92891-4
- H.H. Handsfield, E.G. Sandström, J.S. Knapp, P.L. Perine, W.L. Whittington, D.E. Sayers, and K.K. Holmes, "Epidemiology of Penicillinase-ProducingNeisseria gonorrhoeaeInfections", New England Journal of Medicine, vol. 306, pp. 950-954, 1982. http://dx.doi.org/10.1056/NEJM198204223061602
- H.H. HANDSFIELD, J.S. KNAPP, P.K. DIEHR, and K.K. HOLMES, "Correlation of A uxo type and Penicillin Susceptibility of Neisseria gonorrhoeae with Sexual Preference and Clinical Manifestations of Gonorrhea", Sexually Transmitted Diseases, vol. 7, pp. 1-5, 1980. http://dx.doi.org/10.1097/00007435-198001000-00001
- J.A. Yorke, H.W. Hethcote, and A. Nold, "Dynamics and control of the transmission of gonorrhea.", Sexually transmitted diseases, 1978. http://www.ncbi.nlm.nih.gov/pubmed/10328031
- H.H. Handsfield, "Localized Outbreak of Penicillinase-Producing Neisseria gonorrhoeae", JAMA, vol. 261, pp. 2357, 1989. http://dx.doi.org/10.1001/jama.1989.03420160089028
- J.J. POTTERAT, R. ROTHENBERG, and D.C. BROSS, "Gonorrhea in Street Prostitutes", Sexually Transmitted Diseases, vol. 6, pp. 58-63, 1979. http://dx.doi.org/10.1097/00007435-197904000-00003
- D.M. Upchurch, W.E. Brady, C.A. Reichart, and E.W. Hook, "Behavioral Contributions to Acquisition of Gonorrhea in Patients Attending an Inner City Sexually Transmitted Disease Clinic", Journal of Infectious Diseases, vol. 161, pp. 938-941, 1990. http://dx.doi.org/10.1093/infdis/161.5.938
- R. Platt, "Risk of Acquiring Gonorrhea and Prevalence of Abnormal Adnexal Findings Among Women Recently Exposed to Gonorrhea", JAMA: The Journal of the American Medical Association, vol. 250, pp. 3205, 1983. http://dx.doi.org/10.1001/jama.1983.03340230057031
- . WHO, "Neisseria gonorrhoeae and gonococcal infections.", World Health Organization, Switzerland., 1978.
- B.G. Spratt, and M.C.J. Maiden, "Bacterial population genetics, evolution and epidemiology", Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, vol. 354, pp. 701-710, 1999. http://dx.doi.org/10.1098/rstb.1999.0423
- J.S. Bennett, K.A. Jolley, P.F. Sparling, N.J. Saunders, C.A. Hart, I.M. Feavers, and M.C. Maiden, "Species status of Neisseria gonorrhoeae: evolutionary and epidemiological inferences from multilocus sequence typing", BMC Biology, vol. 5, 2007. http://dx.doi.org/10.1186/1741-7007-5-35
- R.K. Selander, P. Beltran, N.H. Smith, R.M. Barker, P.B. Crichton, D.C. Old, J.M. Musser, and T.S. Whittam, "Genetic population structure, clonal phylogeny, and pathogenicity of Salmonella paratyphi B.", Infection and immunity, 1990. http://www.ncbi.nlm.nih.gov/pubmed/2341183
- R.K. Selander, D.A. Caugant, H. Ochman, J.M. Musser, M.N. Gilmour, and T.S. Whittam, "Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics.", Applied and environmental microbiology, 1986. http://www.ncbi.nlm.nih.gov/pubmed/2425735
- J.M. Smith, N.H. Smith, M. O'Rourke, and B.G. Spratt, "How clonal are bacteria?", Proceedings of the National Academy of Sciences, vol. 90, pp. 4384-4388, 1993. http://dx.doi.org/10.1073/pnas.90.10.4384
- R.K. Selander, and B.R. Levin, "Genetic Diversity and Structure in Escherichia coli Populations", Science, vol. 210, pp. 545-547, 1980. http://dx.doi.org/10.1126/science.6999623
- D.A. Caugant, B.R. Levin, and R.K. Selander, "Genetic diversity and temporal variation in the E. coli population of a human host.", Genetics, 1981. http://www.ncbi.nlm.nih.gov/pubmed/7037535
- E.J. Feil, B.C. Li, D.M. Aanensen, W.P. Hanage, and B.G. Spratt, "eBURST: Inferring Patterns of Evolutionary Descent among Clusters of Related Bacterial Genotypes from Multilocus Sequence Typing Data", Journal of Bacteriology, vol. 186, pp. 1518-1530, 2004. http://dx.doi.org/10.1128/JB.186.5.1518-1530.2004
- H.M. Palmer, and C. Arnold, "Genotyping Neisseria gonorrhoeae Using Fluorescent Amplified Fragment Length Polymorphism Analysis", Journal of Clinical Microbiology, vol. 39, pp. 2325-2329, 2001. http://dx.doi.org/10.1128/JCM.39.6.2325-2329.2001
- E.J. Feil, and B.G. Spratt, "Recombination and the Population Structures of Bacterial Pathogens", Annual Review of Microbiology, vol. 55, pp. 561-590, 2001. http://dx.doi.org/10.1146/annurev.micro.55.1.561
- J.S. Bennett, D.T. Griffiths, N.D. McCarthy, K.L. Sleeman, K.A. Jolley, D.W. Crook, and M.C.J. Maiden, "Genetic Diversity and Carriage Dynamics ofNeisseria lactamicain Infants", Infection and Immunity, vol. 73, pp. 2424-2432, 2005. http://dx.doi.org/10.1128/IAI.73.4.2424-2432.2005
- E.J. Feil, E.C. Holmes, D.E. Bessen, M. Chan, N.P.J. Day, M.C. Enright, R. Goldstein, D.W. Hood, A. Kalia, C.E. Moore, J. Zhou, and B.G. Spratt, "Recombination within natural populations of pathogenic bacteria: Short-term empirical estimates and long-term phylogenetic consequences", Proceedings of the National Academy of Sciences, vol. 98, pp. 182-187, 2001. http://dx.doi.org/10.1073/pnas.98.1.182
- K.A. Jolley, E.J. Feil, M. Chan, and M.C.J. Maiden, "Sequence type analysis and recombinational tests (START)", Bioinformatics, vol. 17, pp. 1230-1231, 2001. http://dx.doi.org/10.1093/bioinformatics/17.12.1230
- M. Pérez-Losada, K.A. Crandall, M.C. Bash, M. Dan, J. Zenilman, and R.P. Viscidi, "Distinguishing importation from diversification of quinolone-resistant Neisseria gonorrhoeae by molecular evolutionary analysis", BMC Evolutionary Biology, vol. 7, 2007. http://dx.doi.org/10.1186/1471-2148-7-84
- S.R. JOHNSON, and S.A. MORSE, "Antibiotic Resistance in Neisseria gonorrhoeae: Genetics and Mechanisms of Resistance", Sexually Transmitted Diseases, vol. 15, pp. 217-224, 1988. http://dx.doi.org/10.1097/00007435-198810000-00008
- S.R. Rohlfing, J.K. Landmesser, J.F. Gerster, E.P. Sharon, and R.M. Stern, "Differentiation of fluorinated quinolone antibacterials with Neisseria gonorrhoeae isolates", Journal of Antimicrobial Chemotherapy, vol. 15, pp. 539-544, 1985. http://dx.doi.org/10.1093/jac/15.5.539
- M. Macaulay, "ACROSOXACIN RESISTANCE IN NEISSERIA GONORRHOEAE", The Lancet, vol. 319, pp. 171-172, 1982. http://dx.doi.org/10.1016/s0140-6736(82)90423-8
- S. Vidovic, S.D. Thakur, G.B. Horsman, P.N. Levett, V. Anvari, and J.R. Dillon, "Longitudinal Analysis of the Evolution and Dissemination of Neisseria gonorrhoeae Strains (Saskatchewan, Canada, 2005 to 2008) Reveals Three Major Circulating Strains and Convergent Evolution of Ciprofloxacin and Azithromycin Resistance", Journal of Clinical Microbiology, vol. 50, pp. 3823-3830, 2012. http://dx.doi.org/10.1128/jcm.01402-12
- B.G. Spratt, W.P. Hanage, B. Li, D.M. Aanensen, and E.J. Feil, "Displaying the relatedness among isolates of bacterial species â the eBURST approach", FEMS Microbiology Letters, vol. 241, pp. 129-134, 2004. http://dx.doi.org/10.1016/j.femsle.2004.11.015
- M. Ohnishi, D. Golparian, K. Shimuta, T. Saika, S. Hoshina, K. Iwasaku, S. Nakayama, J. Kitawaki, and M. Unemo, "Is Neisseria gonorrhoeae Initiating a Future Era of Untreatable Gonorrhea?: Detailed Characterization of the First Strain with High-Level Resistance to Ceftriaxone", Antimicrobial Agents and Chemotherapy, vol. 55, pp. 3538-3545, 2011. http://dx.doi.org/10.1128/aac.00325-11
- M. Unemo, D. Golparian, R. Nicholas, M. Ohnishi, A. Gallay, and P. Sednaoui, "High-Level Cefixime- and Ceftriaxone-Resistant Neisseria gonorrhoeae in France: NovelpenAMosaic Allele in a Successful International Clone Causes Treatment Failure", Antimicrobial Agents and Chemotherapy, vol. 56, pp. 1273-1280, 2012. http://dx.doi.org/10.1128/aac.05760-11
- J. Camara, J. Serra, J. Ayats, T. Bastida, D. Carnicer-Pont, A. Andreu, and C. Ardanuy, "Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain", Journal of Antimicrobial Chemotherapy, vol. 67, pp. 1858-1860, 2012. http://dx.doi.org/10.1093/jac/dks162
- M. Unemo, and R.A. Nicholas, "Emergence of Multidrug-Resistant, Extensively Drug-Resistant and Untreatable Gonorrhea", Future Microbiology, vol. 7, pp. 1401-1422, 2012. http://dx.doi.org/10.2217/fmb.12.117
- M. Unemo, and W.M. Shafer, "Antimicrobial Resistance in Neisseria gonorrhoeae in the 21st Century: Past, Evolution, and Future", Clinical Microbiology Reviews, vol. 27, pp. 587-613, 2014. http://dx.doi.org/10.1128/cmr.00010-14
- M. Unemo, and W.M. Shafer, "Antibiotic resistance inNeisseria gonorrhoeae: origin, evolution, and lessons learned for the future", Annals of the New York Academy of Sciences, vol. 1230, 2011. http://dx.doi.org/10.1111/j.1749-6632.2011.06215.x
- M. Tanaka, H. Tunoe, T. Egashira, S. Naito, H. Nakayama, A. Kanayama, T. Saika, and I. Kobayashi, "A remarkable reduction in the susceptibility of Neisseria gonorrhoeae isolates to cephems and the selection of antibiotic regimens for the single-dose treatment of gonococcal infection in Japan", Journal of Infection and Chemotherapy, vol. 8, pp. 81-86, 2002. http://dx.doi.org/10.1007/s101560200011
- R. Furuya, M. Tanaka, Y. Onoye, A. Kanayama, T. Saika, T. Iyoda, M. Tatewaki, K. Matsuzaki, and I. Kobayashi, "Antimicrobial resistance in clinical isolates of Neisseria subflava from the oral cavities of a Japanese population", Journal of Infection and Chemotherapy, vol. 13, pp. 302-304, 2007. http://dx.doi.org/10.1007/s10156-007-0541-8
- W. ASHFORD, "PENICILUNASE-PRODUCING NEISSERIA GONORRH$OElig;q", The Lancet, vol. 308, pp. 657-658, 1976. http://dx.doi.org/10.1016/s0140-6736(76)92467-3
- I. PHILLIPS, "$bgr;-LACTAMASE-PRODUCING, PENICILLIN-RESISTANT GONOCOCCUS", The Lancet, vol. 308, pp. 656-657, 1976. http://dx.doi.org/10.1016/s0140-6736(76)92466-1
- J.G. Cannon, and P.F. Sparling, "THE GENETICS OF THE GONOCOCCUS", Annual Review of Microbiology, vol. 38, pp. 111-133, 1984. http://dx.doi.org/10.1146/annurev.mi.38.100184.000551
- S.A. Morse, S.R. Johnson, J.W. Biddle, and M.C. Roberts, "High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant", Antimicrobial Agents and Chemotherapy, vol. 30, pp. 664-670, 1986. http://dx.doi.org/10.1128/aac.30.5.664
- T.E. Sox, W. Mohammed, E. Blackman, G. Biswas, and P.F. Sparling, "Conjugative plasmids in Neisseria gonorrhoeae.", Journal of bacteriology, 1978. http://www.ncbi.nlm.nih.gov/pubmed/418060
- B.I. Eisenstein, T. Sox, G. Biswas, E. Blackman, and P.F. Sparling, "Conjugal Transfer of the Gonococcal Penicillinase Plasmid", Science, vol. 195, pp. 998-1000, 1977. http://dx.doi.org/10.1126/science.402693
- L.A. Kirven, and C. Thornsberry, "Transfer of Beta-Lactamase Genes of Neisseria gonorrhoeae by Conjugation", Antimicrobial Agents and Chemotherapy, vol. 11, pp. 1004-1006, 1977. http://dx.doi.org/10.1128/aac.11.6.1004
- A.J. Powell, J. Tomberg, A.M. Deacon, R.A. Nicholas, and C. Davies, "Crystal Structures of Penicillin-binding Protein 2 from Penicillin-susceptible and -resistant Strains of Neisseria gonorrhoeae Reveal an Unexpectedly Subtle Mechanism for Antibiotic Resistance", Journal of Biological Chemistry, vol. 284, pp. 1202-1212, 2009. http://dx.doi.org/10.1074/jbc.m805761200
- P.F. Sparling, F.A. Sarubbi, and E. Blackman, "Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae.", Journal of bacteriology, 1975. http://www.ncbi.nlm.nih.gov/pubmed/810479
- S. Zhao, M. Duncan, J. Tomberg, C. Davies, M. Unemo, and R.A. Nicholas, "Genetics of Chromosomally Mediated Intermediate Resistance to Ceftriaxone and Cefixime in Neisseria gonorrhoeae", Antimicrobial Agents and Chemotherapy, vol. 53, pp. 3744-3751, 2009. http://dx.doi.org/10.1128/AAC.00304-09
- D.M. Warner, W.M. Shafer, and A.E. Jerse, "Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC‐MtrD‐MtrE Efflux pump system confer different levels of antimicrobial resistance and in vivo fitness", Molecular Microbiology, vol. 70, pp. 462-478, 2008. http://dx.doi.org/10.1111/j.1365-2958.2008.06424.x
- D. Warner, J. Folster, W. Shafer, and A. Jerse, "Regulation of the MtrC‐MtrD‐MtrE Efflux‐Pump System Modulates the In Vivo Fitness ofNeisseria gonorrhoeae", The Journal of Infectious Diseases, vol. 196, pp. 1804-1812, 2007. http://dx.doi.org/10.1086/522964
- W.L. Veal, R.A. Nicholas, and W.M. Shafer, "Overexpression of the MtrC-MtrD-MtrE Efflux Pump Due to an mtrR Mutation Is Required for Chromosomally Mediated Penicillin Resistance in Neisseria gonorrhoeae", Journal of Bacteriology, vol. 184, pp. 5619-5624, 2002. http://dx.doi.org/10.1128/JB.184.20.5619-5624.2002
- L. Zarantonelli, G. Borthagaray, E.H. Lee, and W.M. Shafer, "Decreased azithromycin susceptibility of Neisseria gonorrhoeae due to mtrR mutations.", Antimicrobial agents and chemotherapy, 1999. http://www.ncbi.nlm.nih.gov/pubmed/10508026
- D. Golparian, W.M. Shafer, M. Ohnishi, and M. Unemo, "Importance of Multidrug Efflux Pumps in the Antimicrobial Resistance Property of Clinical Multidrug-Resistant Isolates of Neisseria gonorrhoeae", Antimicrobial Agents and Chemotherapy, vol. 58, pp. 3556-3559, 2014. http://dx.doi.org/10.1128/aac.00038-14
- W.M. Shafer, X. Qu, A.J. Waring, and R.I. Lehrer, "Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family", Proceedings of the National Academy of Sciences, vol. 95, pp. 1829-1833, 1998. http://dx.doi.org/10.1073/pnas.95.4.1829
- C. Bignell, M. Unemo, . , K. Radcliffe, J.S. Jensen, K. Babayan, S. Barton, M. Cusini, M. Gomberg, M. Janier, L. Khotenashvili, H. Moi, M. Neumann, R. Patel, J. Ross, J. Sherrard, G. Tiplica, M. Unemo, M. Van De Laar, and W. Van Der, "2012 European guideline on the diagnosis and treatment of gonorrhoea in adults", International Journal of STD & AIDS, vol. 24, pp. 85-92, 2013. http://dx.doi.org/10.1177/0956462412472837
- "Update to CDC’s <emph type="ital">Sexually Transmitted Diseases Treatment Guidelines, 2010</emph>: Oral Cephalosporins No Longer a Recommended Treatment for Gonococcal Infections", JAMA, vol. 308, pp. 1850, 2012. http://dx.doi.org/10.1001/2012.jama.11292
- R.D. Kirkcaldy, H.S. Weinstock, P.C. Moore, S.S. Philip, H.C. Wiesenfeld, J.R. Papp, P.R. Kerndt, S. Johnson, K.G. Ghanem, E.W. Hook, L.M. Newman, D. Dowell, C. Deal, J. Glock, L. Venkatasubramanian, L. McNeil, C. Perlowski, J.Y. Lee, S. Lensing, N. Trainor, S. Fuller, A. Herrera, J.S. Carlson, H. Harbison, C. Lenderman, P. Dixon, A. Whittington, I. Macio, C. Priest, A. Jett, T. Campbell, A. Uniyal, L. Royal, M. Mejia, J. Vonghack, S. Tobias, J. Zenilman, J. Long, A. Harvey, K. Pettus, and S. Sharpe, "The Efficacy and Safety of Gentamicin Plus Azithromycin and Gemifloxacin Plus Azithromycin as Treatment of Uncomplicated Gonorrhea", Clinical Infectious Diseases, vol. 59, pp. 1083-1091, 2014. http://dx.doi.org/10.1093/cid/ciu521
- C.A. Ison, C. Deal, and M. Unemo, "Current and future treatment options for gonorrhoea", Sexually Transmitted Infections, vol. 89, pp. iv52-iv56, 2013. http://dx.doi.org/10.1136/sextrans-2012-050913
- L.B. Brown, R. Krysiak, G. Kamanga, C. Mapanje, H. Kanyamula, B. Banda, C. Mhango, M. Hoffman, D. Kamwendo, M. Hobbs, M.C. Hosseinipour, F. Martinson, M.S. Cohen, and I.F. Hoffman, "Neisseria gonorrhoeae Antimicrobial Susceptibility in Lilongwe, Malawi, 2007", Sexually Transmitted Diseases, vol. 37, pp. 169-172, 2010. http://dx.doi.org/10.1097/olq.0b013e3181bf575c
- D. Golparian, P. Fernandes, M. Ohnishi, J.S. Jensen, and M. Unemo, "In Vitro Activity of the New Fluoroketolide Solithromycin (CEM-101) against a Large Collection of Clinical Neisseria gonorrhoeae Isolates and International Reference Strains, Including Those with High-Level Antimicrobial Resistance: Potential Treatment Option for Gonorrhea?", Antimicrobial Agents and Chemotherapy, vol. 56, pp. 2739-2742, 2012. http://dx.doi.org/10.1128/aac.00036-12
- M. Unemo, D. Golparian, A. Limnios, D. Whiley, M. Ohnishi, M.M. Lahra, and J.W. Tapsall, "In VitroActivity of Ertapenem versus Ceftriaxone against Neisseria gonorrhoeae Isolates with Highly Diverse Ceftriaxone MIC Values and Effects of Ceftriaxone Resistance Determinants: Ertapenem for Treatment of Gonorrhea?", Antimicrobial Agents and Chemotherapy, vol. 56, pp. 3603-3609, 2012. http://dx.doi.org/10.1128/aac.00326-12
- S. Jacobsson, D. Golparian, L.T. Phan, M. Ohnishi, H. Fredlund, Y.S. Or, and M. Unemo, "In vitro activities of the novel bicyclolides modithromycin (EDP-420, EP-013420, S-013420) and EDP-322 against MDR clinical Neisseria gonorrhoeae isolates and international reference strains", Journal of Antimicrobial Chemotherapy, vol. 70, pp. 173-177, 2014. http://dx.doi.org/10.1093/jac/dku344
- M. Falagas, D. Karageorgopoulos, and G. Dimopoulos, "Clinical Significance of the Pharmacokinetic and Pharmacodynamic Characteristics of Tigecycline", Current Drug Metabolism, vol. 10, pp. 13-21, 2009. http://dx.doi.org/10.2174/138920009787048356
- D.E. Nix, and K.R. Matthias, "Should tigecycline be considered for urinary tract infections? A pharmacokinetic re-evaluation", Journal of Antimicrobial Chemotherapy, vol. 65, pp. 1311-1312, 2010. http://dx.doi.org/10.1093/jac/dkq116
- D.J. Biedenbach, L.L. Turner, R.N. Jones, and D.J. Farrell, "Activity of JNJ-Q2, a novel fluoroquinolone, tested against Neisseria gonorrhoeae, including ciprofloxacin-resistant strains", Diagnostic Microbiology and Infectious Disease, vol. 74, pp. 204-206, 2012. http://dx.doi.org/10.1016/j.diagmicrobio.2012.06.006
- R. Hamasuna, M. Yasuda, K. Ishikawa, S. Uehara, H. Hayami, S. Takahashi, T. Matsumoto, S. Yamamoto, S. Minamitani, A. Watanabe, S. Iwata, M. Kaku, J. Kadota, K. Sunakawa, J. Sato, H. Hanaki, T. Tsukamoto, H. Kiyota, S. Egawa, K. Tanaka, S. Arakawa, M. Fujisawa, H. Kumon, K. Kobayashi, A. Matsubara, S. Naito, K. Kuroiwa, H. Hirayama, H. Narita, T. Hosobe, S. Ito, K. Ito, S. Kawai, M. Ito, H. Chokyu, M. Matsumura, M. Yoshioka, S. Uno, K. Monden, K. Takayama, S. Kaji, M. Kawahara, T. Sumii, H. Kadena, T. Yamaguchi, S. Maeda, S. Nishi, H. Nishimura, T. Shirane, M. Yoh, K. Akiyama, T. Imai, and M. Kano, "The second nationwide surveillance of the antimicrobial susceptibility of Neisseria gonorrhoeae from male urethritis in Japan, 2012–2013", Journal of Infection and Chemotherapy, vol. 21, pp. 340-345, 2015. http://dx.doi.org/10.1016/j.jiac.2015.01.010
- D. Kazamori, H. Aoi, K. Sugimoto, T. Ueshima, H. Amano, K. Itoh, Y. Kuramoto, and A. Yazaki, "In vitro activity of WQ-3810, a novel fluoroquinolone, against multidrug-resistant and fluoroquinolone-resistant pathogens", International Journal of Antimicrobial Agents, vol. 44, pp. 443-449, 2014. http://dx.doi.org/10.1016/j.ijantimicag.2014.07.017
- K. Fujimoto, K. Takemoto, K. Hatano, T. Nakai, S. Terashita, M. Matsumoto, Y. Eriguchi, K. Eguchi, T. Shimizudani, K. Sato, K. Kanazawa, M. Sunagawa, and Y. Ueda, "Novel Carbapenem Antibiotics for Parenteral and Oral Applications: In Vitro and In Vivo Activities of 2-Aryl Carbapenems and Their Pharmacokinetics in Laboratory Animals", Antimicrobial Agents and Chemotherapy, vol. 57, pp. 697-707, 2013. http://dx.doi.org/10.1128/aac.01051-12
- R.E. Mendes, M.R.K. Alley, H.S. Sader, D.J. Biedenbach, and R.N. Jones, "Potency and Spectrum of Activity of AN3365, a Novel Boron-Containing Protein Synthesis Inhibitor, Tested against Clinical Isolates of Enterobacteriaceae and Nonfermentative Gram-Negative Bacilli", Antimicrobial Agents and Chemotherapy, vol. 57, pp. 2849-2857, 2013. http://dx.doi.org/10.1128/aac.00160-13
- O. Lomovskaya, and W. Watkins, "Inhibition of efflux pumps as a novel approach to combat drug resistance in bacteria.", Journal of molecular microbiology and biotechnology, 2001. http://www.ncbi.nlm.nih.gov/pubmed/11321578
- L. Li, M. Yen, C. Ho, P. Wu, C. Wang, P.K. Maurya, P. Chen, W. Chen, W. Hsieh, and H. Chen, "Non-Cytotoxic Nanomaterials Enhance Antimicrobial Activities of Cefmetazole against Multidrug-Resistant Neisseria gonorrhoeae", PLoS ONE, vol. 8, pp. e64794, 2013. http://dx.doi.org/10.1371/journal.pone.0064794
- R. Bucki, K. Leszczyńska, A. Namiot, and W. Sokołowski, "Cathelicidin LL-37: A Multitask Antimicrobial Peptide", Archivum Immunologiae et Therapiae Experimentalis, vol. 58, pp. 15-25, 2010. http://dx.doi.org/10.1007/s00005-009-0057-2
- P. Zhou, and A. Barb, "Mechanism and Inhibition of LpxC: An Essential Zinc-Dependent Deacetylase of Bacterial Lipid A Synthesis", Current Pharmaceutical Biotechnology, vol. 9, pp. 9-15, 2008. http://dx.doi.org/10.2174/138920108783497668
- S. Escaich, L. Prouvensier, M. Saccomani, L. Durant, M. Oxoby, V. Gerusz, F. Moreau, V. Vongsouthi, K. Maher, I. Morrissey, and C. Soulama-Mouze, "The MUT056399 Inhibitor of FabI Is a New Antistaphylococcal Compound", Antimicrobial Agents and Chemotherapy, vol. 55, pp. 4692-4697, 2011. http://dx.doi.org/10.1128/aac.01248-10
- S. Jeverica, D. Golparian, B. Hanzelka, A.J. Fowlie, M. Mati i , and M. Unemo, "High in vitro activity of a novel dual bacterial topoisomerase inhibitor of the ATPase activities of GyrB and ParE (VT12-008911) against Neisseria gonorrhoeae isolates with various high-level antimicrobial resistance and multidrug resistance", Journal of Antimicrobial Chemotherapy, vol. 69, pp. 1866-1872, 2014. http://dx.doi.org/10.1093/jac/dku073
- M. Unemo, and W.M. Shafer, "Future treatment of gonorrhoea – novel emerging drugs are essential and in progress?", Expert Opinion on Emerging Drugs, vol. 20, pp. 357-360, 2015. http://dx.doi.org/10.1517/14728214.2015.1039981
- G. Kern, T. Palmer, D.E. Ehmann, A.B. Shapiro, B. Andrews, G.S. Basarab, P. Doig, J. Fan, N. Gao, S.D. Mills, J. Mueller, S. Sriram, J. Thresher, and G.K. Walkup, "Inhibition of Neisseria gonorrhoeae Type II Topoisomerases by the Novel Spiropyrimidinetrione AZD0914", Journal of Biological Chemistry, vol. 290, pp. 20984-20994, 2015. http://dx.doi.org/10.1074/jbc.m115.663534
- R.A. Alm, S.D. Lahiri, A. Kutschke, L.G. Otterson, R.E. McLaughlin, J.D. Whiteaker, L.A. Lewis, X. Su, M.D. Huband, H. Gardner, and J.P. Mueller, "Characterization of the Novel DNA Gyrase Inhibitor AZD0914: Low Resistance Potential and Lack of Cross-Resistance in Neisseria gonorrhoeae", Antimicrobial Agents and Chemotherapy, vol. 59, pp. 1478-1486, 2015. http://dx.doi.org/10.1128/aac.04456-14
- N. Goire, M.M. Lahra, M. Chen, B. Donovan, C.K. Fairley, R. Guy, J. Kaldor, D. Regan, J. Ward, M.D. Nissen, T.P. Sloots, and D.M. Whiley, "Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance", Nature Reviews Microbiology, vol. 12, pp. 223-229, 2014. http://dx.doi.org/10.1038/nrmicro3217
ACKNOWLEDGMENTS
Dedicated to the memory of John Swanson.
COPYRIGHT
© 2016

Gonorrhea – an evolving disease of the new millennium by Hill et al. is licensed under a Creative Commons Attribution 4.0 International License.









