Back to article: Methodologies for in vitro and in vivo evaluation of efficacy of antifungal and antibiofilm agents and surface coatings against fungal biofilms
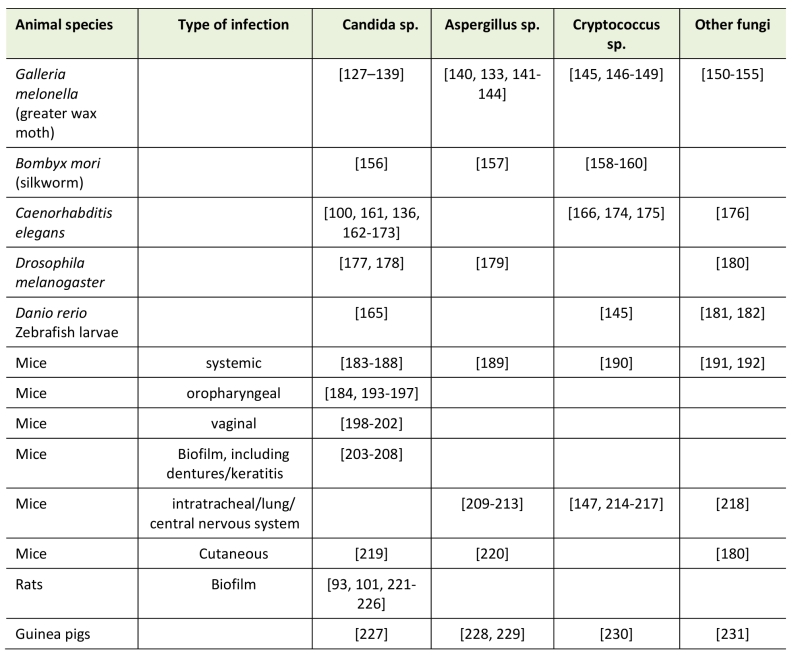
TABLE 1. Overview of in vivo models for assessing efficacy of antifungal drugs or treatments [refer-ences].
93. Cools TL, Struyfs C, Drijfhout JW, Kucharíková S, Lobo Romero C, Van Dijck P, Ramada MHS, Bloch C, Cammue BPA, and Thevissen K (2017). A Linear 19-Mer Plant Defensin-Derived Peptide Acts Synergistically with Caspofungin againstCandida albicansBiofilms. Front Microbiol 8: 2051. http://dx.doi.org/10.3389/fmicb.2017.02051
100. Delattin N, De Brucker K, Vandamme K, Meert E, Marchand A, Chaltin P, Cammue BPA, and Thevissen K (2014). Repurposing as a means to increase the activity of amphotericin B and caspofungin against Candida albicans biofilms. J Antimicrob Chemother 69(4): 1035–44. http://dx.doi.org/10.1093/jac/dkt449
101. De Cremer K, Delattin N, De Brucker K, Peeters A, Kucharíková S, Gerits E, Verstraeten N, Michiels J, Van Dijck P, Cammue BPA, and Thevissen K (2014). Oral administration of the broad-spectrum antibiofilm compound toremifene inhibits Candida albicans and Staphylococcus aureus biofilm formation in vivo. Antimicrob Agents Chemother 58(12): 7606–10. http://dx.doi.org/10.1128/AAC.03869-14
127. Gu W, Yu Q, Yu C, and Sun S (2017). In vivo activity of fluconazole/tetracycline combinations in Galleria mellonella with resistant Candida albicans infection. J Glob Antimicrob Resist 13:74-80. http://dx.doi.org/10.1016/j.jgar.2017.11.011
128. Ames L, Duxbury S, Pawlowska B, Ho H-L, Haynes K, and Bates S (2017). Galleria mellonella as a host model to study Candida glabrata virulence and antifungal efficacy. Virulence 8(8): 1909–1917. http://dx.doi.org/10.1080/21505594.2017.1347744
129. Aneja B, Irfan M, Kapil C, Jairajpuri MA, Maguire R, Kavanagh K, Rizvi MMA, Manzoor N, Azam A, and Abid M (2016). Effect of novel triazole–amino acid hybrids on growth and virulence of Candida species: in vitro and in vivo studies. Org Biomol Chem 14(45): 10599–10619. http://dx.doi.org/10.1039/C6OB01718E
130. Lu M, Yu C, Cui X, Shi J, Yuan L, and Sun S (2018). Gentamicin synergises with azoles against drug-resistant Candida albicans. Int J Antimicrob Agents 51(1): 107–114. http://dx.doi.org/10.1016/j.ijantimicag.2017.09.012
131. Souza ACR, Fuchs BB, Pinhati HMS, Siqueira RA, Hagen F, Meis JF, Mylonakis E, and Colombo AL (2015). Candida parapsilosis Resistance to Fluconazole: Molecular Mechanisms and In Vivo Impact in Infected Galleria mellonella Larvae. Antimicrob Agents Chemother 59(10): 6581–7. http://dx.doi.org/10.1128/AAC.01177-15
132. Lopez-Moya F, Colom-Valiente MF, Martinez-Peinado P, Martinez-Lopez JE, Puelles E, Sempere-Ortells JM, and Lopez-Llorca LV (2015). Carbon and nitrogen limitation increase chitosan antifungal activity in Neurospora crassa and fungal human pathogens. Fungal Biol 119(2–3): 154–69. http://dx.doi.org/10.1016/j.funbio.2014.12.003
133. Maurer E, Browne N, Surlis C, Jukic E, Moser P, Kavanagh K, Lass-Flörl C, and Binder U (2015). Galleria mellonella as a host model to study Aspergillus terreus virulence and amphotericin B resistance. Virulence 6(6): 591–8. http://dx.doi.org/10.1080/21505594.2015.1045183
134. Favre-Godal Q, Dorsaz S, Queiroz EF, Conan C, Marcourt L, Wardojo BPE, Voinesco F, Buchwalder A, Gindro K, Sanglard D, and Wolfender J-L (2014). Comprehensive approach for the detection of antifungal compounds using a susceptible strain of Candida albicans and confirmation of in vivo activity with the Galleria mellonella model. Phytochemistry 105: 68–78. http://dx.doi.org/10.1016/j.phytochem.2014.06.004
135. Li D-D, Deng L, Hu G-H, Zhao L-X, Hu D-D, Jiang Y-Y, and Wang Y (2013). Using Galleria mellonella-Candida albicans infection model to evaluate antifungal agents. Biol Pharm Bull 36(9): 1482–7. https://www.ncbi.nlm.nih.gov/pubmed/?term=23995660
136. Scorzoni L, de Lucas MP, Mesa-Arango AC, Fusco-Almeida AM, Lozano E, Cuenca-Estrella M, Mendes-Giannini MJ, and Zaragoza O (2013). Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PLoS One 8(3): e60047. http://dx.doi.org/10.1371/journal.pone.0060047
137. Mesa-Arango AC, Forastiero A, Bernal-Martínez L, Cuenca-Estrella M, Mellado E, and Zaragoza O (2013). The non-mammalian host Galleria mellonella can be used to study the virulence of the fungal pathogen Candida tropicalis and the efficacy of antifungal drugs during infection by this pathogenic yeast. Med Mycol 51(5): 461–72. http://dx.doi.org/10.3109/13693786.2012.737031
138. Rowan R, Moran C, McCann M, and Kavanagh K (2009). Use of Galleria mellonella larvae to evaluate the in vivo anti-fungal activity of [Ag2(mal)(phen)3]. Biometals 22(3): 461–7. http://dx.doi.org/10.1007/s10534-008-9182-3
139. Delarze E, Ischer F, Sanglard D, and Coste AT (2015). Adaptation of a Gaussia princeps Luciferase reporter system in Candida albicans for in vivo detection in the Galleria mellonella infection model. Virulence 6(7): 684–693. http://dx.doi.org/10.1080/21505594.2015.1081330
140. Forastiero A, Bernal-Martínez L, Mellado E, Cendejas E, and Gomez-Lopez A (2015). In vivo efficacy of voriconazole and posaconazole therapy in a novel invertebrate model of Aspergillus fumigatus infection. Int J Antimicrob Agents 46(5): 511–7. http://dx.doi.org/10.1016/j.ijantimicag.2015.07.007
141. Ben Yaakov D, Rivkin A, Mircus G, Albert N, Dietl A-M, Kovalerchick D, Carmeli S, Haas H, Kontoyiannis DP, and Osherov N (2016). Identification and characterization of haemofungin, a novel antifungal compound that inhibits the final step of haem biosynthesis. J Antimicrob Chemother 71(4): 946–52. http://dx.doi.org/10.1093/jac/dkv446
142. Ben Yaakov D, Shadkchan Y, Albert N, Kontoyiannis DP, and Osherov N (2017). The quinoline bromoquinol exhibits broad-spectrum antifungal activity and induces oxidative stress and apoptosis in Aspergillus fumigatus. J Antimicrob Chemother 72(8): 2263–2272. http://dx.doi.org/10.1093/jac/dkx117
143. Alcazar-Fuoli L, Buitrago M, Gomez-Lopez A, and Mellado E (2015). An alternative host model of a mixed fungal infection by azole susceptible and resistant Aspergillus spp strains. Virulence 6(4): 376–384. http://dx.doi.org/10.1080/21505594.2015.1025192
144. Gomez-Lopez A, Forastiero A, Cendejas-Bueno E, Gregson L, Mellado E, Howard SJ, Livermore JL, Hope WW, and Cuenca-Estrella M (2014). An invertebrate model to evaluate virulence in Aspergillus fumigatus: the role of azole resistance. Med Mycol 52(3): 311–9. http://dx.doi.org/10.1093/mmy/myt022
145. Palanco AC, Lacorte Singulani J de, Costa-Orlandi CB, Gullo FP, Strohmayer Lourencetti NM, Gomes PC, Ayusso GM, Dutra LA, Silva Bolzani V da, Regasini LO, Soares Mendes-Giannini MJ, and Fusco-Almeida AM (2017). Activity of 3’-hydroxychalcone against Cryptococcus gattii and toxicity, and efficacy in alternative animal models. Future Microbiol 12(13): 1123–1134. http://dx.doi.org/10.2217/fmb-2017-0062
146. de Sá NP, de Barros PP, Junqueira JC, Vaz JA, de Oliveira RB, Rosa CA, Santos DA, and Johann S (2018). Thiazole derivatives act on virulence factors of Cryptococcus spp. Med Mycol . http://dx.doi.org/10.1093/mmy/myx158
147. Sangalli-Leite F, Scorzoni L, Alves de Paula E Silva AC, da Silva J de F, de Oliveira HC, de Lacorte Singulani J, Gullo FP, Moraes da Silva R, Regasini LO, Siqueira da Silva DH, da Silva Bolzani V, Fusco-Almeida AM, and Soares Mendes-Giannini MJ (2016). Synergistic effect of pedalitin and amphotericin B against Cryptococcus neoformans by in vitro and in vivo evaluation. Int J Antimicrob Agents 48(5): 504–511. http://dx.doi.org/10.1016/j.ijantimicag.2016.07.025
148. Vu K, and Gelli A (2010). Astemizole and an analogue promote fungicidal activity of fluconazole against Cryptococcus neoformans var. grubii and Cryptococcus gattii. Med Mycol 48(2): 255–62. http://dx.doi.org/10.1080/13693780903081968
149. Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, Calderwood SB, Ausubel FM, and Diener A (2005). Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun 73(7): 3842–50. http://dx.doi.org/10.1128/IAI.73.7.3842-3850.2005
150. Kloezen W, Parel F, Brüggemann R, Asouit K, Helvert-van Poppel M, Fahal A, Mouton J, and van de Sande W (2018). Amphotericin B and terbinafine but not the azoles prolong survival in Galleria mellonella larvae infected with Madurella mycetomatis. Med Mycol 56(4):469-478. http://dx.doi.org/10.1093/mmy/myx064
151. de Lacorte Singulani J, Scorzoni L, de Paula e Silva ACA, Fusco-Almeida AM, and Mendes-Giannini MJS (2016). Evaluation of the efficacy of antifungal drugs against Paracoccidioides brasiliensis and Paracoccidioides lutzii in a Galleria mellonella model. Int J Antimicrob Agents 48(3): 292–297. http://dx.doi.org/10.1016/j.ijantimicag.2016.05.012
152. Kloezen W, van Helvert-van Poppel M, Fahal AH, and van de Sande WWJ (2015). A Madurella mycetomatis Grain Model in Galleria mellonella Larvae. PLoS Negl Trop Dis 9(7): e0003926. http://dx.doi.org/10.1371/journal.pntd.0003926
153. Mariné M, Bom VLP, de Castro PA, Winkelstroter LK, Ramalho LN, Brown NA, and Goldman GH (2015). The development of animal infection models and antifungal efficacy assays against clinical isolates of Trichosporon asahii , T. asteroides and T. inkin. Virulenc 6(5): 476–486. http://dx.doi.org/10.1080/21505594.2015.1020273
154. Bastidas RJ, Shertz CA, Lee SC, Heitman J, and Cardenas ME (2012). Rapamycin exerts antifungal activity in vitro and in vivo against Mucor circinelloides via FKBP12-dependent inhibition of Tor. Eukaryot Cell 11(3): 270–81. http://dx.doi.org/10.1128/EC.05284-11
155. Coleman JJ, Muhammed M, Kasperkovitz P V, Vyas JM, and Mylonakis E (2011). Fusarium pathogenesis investigated using Galleria mellonella as a heterologous host. Fungal Biol 115(12): 1279–89. http://dx.doi.org/10.1016/j.funbio.2011.09.005
156. Uchida R, Namiguchi S, Ishijima H, and Tomoda H (2016). Therapeutic effects of three trichothecenes in the silkworm infection assay with Candida albicans. Drug Discov Ther 10(1): 44–8. http://dx.doi.org/10.5582/ddt.2016.01013
157. Nakamura I, Kanasaki R, Yoshikawa K, Furukawa S, Fujie A, Hamamoto H, and Sekimizu K (2017). Discovery of a new antifungal agent ASP2397 using a silkworm model of Aspergillus fumigatus infection. J Antibiot 70(1): 41–44. http://dx.doi.org/10.1038/ja.2016.106
158. Matsumoto Y, Ishii M, Shimizu K, Kawamoto S, and Sekimizu K (2017). A Silkworm Infection Model to Evaluate Antifungal Drugs for Cryptococcosis. Med Mycol J 58(4): E131–E137. http://dx.doi.org/10.3314/mmj.17.016
159. Ishii M, Matsumoto Y, and Sekimizu K (2016). Usefulness of silkworm as a host animal for understanding pathogenicity of Cryptococcus neoformans. Drug Discov Ther 10(1): 9–13. http://dx.doi.org/10.5582/ddt.2016.01015
160. Matsumoto Y, Miyazaki S, Fukunaga DH, Shimizu K, Kawamoto S, and Sekimizu K (2012). Quantitative evaluation of cryptococcal pathogenesis and antifungal drugs using a silkworm infection model with Cryptococcus neoformans. J Appl Microbiol 112(1): 138–46. http://dx.doi.org/10.1111/j.1365-2672.2011.05186.x
161. Muhammed M, Arvanitis M, and Mylonakis E (2016). Whole animal HTS of small molecules for antifungal compounds. Expert Opin Drug Discov 11(2): 177–84. http://dx.doi.org/10.1517/17460441.2016.1122591
162. Sun L, Liao K, and Hang C (2018). Caffeic acid phenethyl ester synergistically enhances the antifungal activity of fluconazole against resistant Candida albicans. Phytomedicine 40: 55–58. http://dx.doi.org/10.1016/j.phymed.2017.12.033
163. Subramenium GA, Swetha TK, Iyer PM, Balamurugan K, and Pandian SK (2018). 5-hydroxymethyl-2-furaldehyde from marine bacterium Bacillus subtilis inhibits biofilm and virulence of Candida albicans. Microbiol Res 207: 19–32. http://dx.doi.org/10.1016/j.micres.2017.11.002
164. Mohammad H, Elghazawy NH, Eldesouky HE, Hegazy YA, Younis W, Avrimova L, Hazbun T, Arafa RK, and Seleem MN (2018). Discovery of a Novel Dibromoquinoline Compound Exhibiting Potent Antifungal and Antivirulence Activity That Targets Metal Ion Homeostasis. ACS Infect Dis 4(3): 403–414. http://dx.doi.org/10.1021/acsinfecdis.7b00215
165. Singulani J de L, Scorzoni L, Gomes PC, Nazaré AC, Polaquini CR, Regasini LO, Fusco-Almeida AM, and Mendes-Giannini MJS (2017). Activity of gallic acid and its ester derivatives in Caenorhabditis elegans and zebrafish (Danio rerio) models. Future Med Chem 9(16): 1863–1872. http://dx.doi.org/10.4155/fmc-2017-0096
166. Thangamani S, Maland M, Mohammad H, Pascuzzi PE, Avramova L, Koehler CM, Hazbun TR, and Seleem MN (2017). Repurposing Approach Identifies Auranofin with Broad Spectrum Antifungal Activity That Targets Mia40-Erv1 Pathway. Front Cell Infect Microbiol 7: 4. http://dx.doi.org/10.3389/fcimb.2017.00004
167. Shu C, Sun L, and Zhang W (2016). Thymol has antifungal activity against Candida albicans during infection and maintains the innate immune response required for function of the p38 MAPK signaling pathway in Caenorhabditis elegans. Immunol Res 64(4): 1013–24. http://dx.doi.org/10.1007/s12026-016-8785-y
168. Li Y, Chang W, Zhang M, Ying Z, and Lou H (2015). Natural product solasodine-3-O-β-D-glucopyranoside inhibits the virulence factors of Candida albicans. FEMS Yeast Res 15(6): fov060. http://dx.doi.org/10.1093/femsyr/fov060
169. Sun L, Liao K, and Wang D (2015). Effects of Magnolol and Honokiol on Adhesion, Yeast-Hyphal Transition, and Formation of Biofilm by Candida albicans. PLoS One 10(2): e0117695. http://dx.doi.org/10.1371/journal.pone.0117695
170. Vediyappan G, Dumontet V, Pelissier F, and d’Enfert C (2013). Gymnemic acids inhibit hyphal growth and virulence in Candida albicans. PLoS One 8(9): e74189. http://dx.doi.org/10.1371/journal.pone.0074189
171. Okoli I, Coleman JJ, Tampakakis E, Tempakakis E, An WF, Holson E, Wagner F, Conery AL, Larkins-Ford J, Wu G, Stern A, Ausubel FM, and Mylonakis E (2009). Identification of antifungal compounds active against Candida albicans using an improved high-throughput Caenorhabditis elegans assay. PLoS One 4(9): e7025. http://dx.doi.org/10.1371/journal.pone.0007025
172. Tampakakis E, Okoli I, and Mylonakis E (2008). A C. elegans-based, whole animal, in vivo screen for the identification of antifungal compounds. Nat Protoc 3(12): 1925–1931. http://dx.doi.org/10.1038/nprot.2008.193
173. Breger J, Fuchs BB, Aperis G, Moy TI, Ausubel FM, and Mylonakis E (2007). Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog 3(2): e18. http://dx.doi.org/10.1371/journal.ppat.0030018
174. Thangamani S, Eldesouky HE, Mohammad H, Pascuzzi PE, Avramova L, Hazbun TR, and Seleem MN (2017). Ebselen exerts antifungal activity by regulating glutathione (GSH) and reactive oxygen species (ROS) production in fungal cells. Biochim Biophys Acta 1861(1 Pt A): 3002–3010.http://dx.doi.org/10.1016/j.bbagen.2016.09.029
175. Cordeiro R de A, Evangelista AJ de J, Serpa R, Marques FJ de F, de Melo CVS, de Oliveira JS, Franco J da S, de Alencar LP, Bandeira T de JPG, Brilhante RSN, Sidrim JJC, and Rocha MFG (2016). Inhibition of heat-shock protein 90 enhances the susceptibility to antifungals and reduces the virulence of Cryptococcus neoformans/Cryptococcus gattii species complex. Microbiology 162(2): 309–17.http://dx.doi.org/10.1099/mic.0.000222
176. Huang X, Li D, Xi L, and Mylonakis E (2014). Caenorhabditis elegans: A Simple Nematode Infection Model for Penicillium marneffei. PLoS One 9(9): e108764.http://dx.doi.org/10.1371/journal.pone.0108764
177. Glittenberg MT, Silas S, MacCallum DM, Gow NAR, and Ligoxygakis P (2011). Glittenberg MT, Silas S, MacCallum DM, Gow NAR, and Ligoxygakis P. Dis Model Mech 4(4): 504–14.http://dx.doi.org/10.1242/dmm.006619
178. Zanette RA, and Kontoyiannis DP (2013). Paradoxical effect to caspofungin in Candida species does not confer survival advantage in a Drosophila model of candidiasis. Virulence 4(6): 497–8.http://dx.doi.org/10.4161/viru.25523
179. Lionakis MS, and Kontoyiannis DP (2012). Drosophila melanogaster as a Model Organism for Invasive Aspergillosis. Methods Mol Biol 845:455-68.http://dx.doi.org/10.1007/978-1-61779-539-8_32
180. Lewis RE, Ben-Ami R, Best L, Albert N, Walsh TJ, and Kontoyiannis DP (2013). Tacrolimus enhances the potency of posaconazole against Rhizopus oryzae in vitro and in an experimental model of mucormycosis. J Infect Dis 207(5): 834–41.http://dx.doi.org/10.1093/infdis/jis767
181. Dananjaya SHS, Erandani WKCU, Kim C-H, Nikapitiya C, Lee J, and De Zoysa M (2017). Comparative study on antifungal activities of chitosan nanoparticles and chitosan silver nano composites against Fusarium oxysporum species complex . Int J Biol Macromol 105(Pt 1): 478–488.http://dx.doi.org/10.1016/j.ijbiomac.2017.07.056
182. Dananjaya SHS, Udayangani RMC, Shin SY, Edussuriya M, Nikapitiya C, Lee J, and De Zoysa M (2017). In vitro and in vivo antifungal efficacy of plant based lawsone against Fusarium oxysporum species complex. Microbiol Res 201: 21–29.http://dx.doi.org/10.1016/j.micres.2017.04.011
183. Li H, Gong H, Qi Y, Li J, Ji X, Sun J, Tian R, Bao H, Song X, Chen Q, and Liu G (2017). In vitro and in vivo antifungal activities and mechanism of heteropolytungstates against Candida species. Sci Rep 7(1): 16942.http://dx.doi.org/10.1038/s41598-017-17239-8
184. Dorsaz S, Coste AT, and Sanglard D (2017). Red-Shifted Firefly Luciferase Optimized for Candida albicans In vivo Bioluminescence Imaging. Front Microbiol 8: 1478.http://dx.doi.org/10.3389/fmicb.2017.01478
185. Lepak AJ, Zhao M, VanScoy B, Ambrose PG, and Andes DR (2017). Pharmacodynamics of a Long-Acting Echinocandin, CD101, in a Neutropenic Invasive-Candidiasis Murine Model Using an Extended-Interval Dosing Design. Antimicrob Agents Chemother 62(2): e02154-17.http://dx.doi.org/10.1128/AAC.02154-17
186. Li R, Zhang L, Zhang H, Yi Y, Wang L, Chen L, and Zhang L (2017). Protective effect of a novel antifungal peptide derived from human chromogranin a on the immunity of mice infected with Candida krusei. Exp Ther Med 13(5): 2429–2434.http://dx.doi.org/10.3892/etm.2017.4290
187. Wring SA, Randolph R, Park S, Abruzzo G, Chen Q, Flattery A, Garrett G, Peel M, Outcalt R, Powell K, Trucksis M, Angulo D, and Borroto-Esoda K (2017). Preclinical Pharmacokinetics and Pharmacodynamic Target of SCY-078, a First-in-Class Orally Active Antifungal Glucan Synthesis Inhibitor, in Murine Models of Disseminated Candidiasis. Antimicrob Agents Chemother 61(4): e02068-16.http://dx.doi.org/10.1128/AAC.02068-16
188. Wong SSW, Kao RYT, Yuen KY, Wang Y, Yang D, Samaranayake LP, and Seneviratne CJ (2014). In vitro and in vivo activity of a novel antifungal small molecule against Candida infections. PLoS One 9(1): e85836.http://dx.doi.org/10.1371/journal.pone.0085836
189. Paulussen C, Boulet G, Bosschaerts T, Cos P, Fortin A, and Maes L (2015). Efficacy of oleylphosphocholine (OlPC) in vitro and in a mouse model of invasive aspergillosis. Mycoses 58(3): 127–32.http://dx.doi.org/10.1111/myc.12286
190. Rathore SS, Isravel M, Vellaisamy S, Chellappan DR, Cheepurupalli L, Raman T, and Ramakrishnan J (2017). Exploration of Antifungal and Immunomodulatory Potentials of a Furanone Derivative to Rescue Disseminated Cryptococosis in Mice. Sci Rep 7(1): 15400.http://dx.doi.org/10.1038/s41598-017-15500-8
191. Thomson P, López-Fernández L, Guarro J, and Capilla J (2017) Virulence and antifungal therapy of murine disseminated infection by Rhodotorula mucilaginosa. Diagn Microbiol Infect Dis 89(1): 47–51.http://dx.doi.org/10.1016/j.diagmicrobio.2017.06.005
192. Ishida K, Castro RA, Torrado JJ, Serrano DR, Borba-Santos LP, Quintella LP, de Souza W, Rozental S, and Lopes-Bezerra LM (2017). Efficacy of a poly-aggregated formulation of amphotericin B in treating systemic sporotrichosis caused by Sporothrix brasiliensis. Med Mycol 56(3):288-296.http://dx.doi.org/10.1093/mmy/myx040
193. Seleem D, Benso B, Noguti J, Pardi V, and Murata RM (2016). In Vitro and In Vivo Antifungal Activity of Lichochalcone-A against Candida albicans Biofilms. PLoS One 11(6): e0157188.http://dx.doi.org/10.1371/journal.pone.0157188
194. Break TJ, Desai J V, Natarajan M, Ferre EMN, Henderson C, Zelazny AM, Siebenlist U, Hoekstra WJ, Schotzinger RJ, Garvey EP, and Lionakis MS (2018. VT-1161 protects mice against oropharyngeal candidiasis caused by fluconazole-susceptible and -resistant Candida albicans. J Antimicrob Chemother 73(1): 151–155.http://dx.doi.org/10.1093/jac/dkx352
195. Vila TVM, Chaturvedi AK, Rozental S, and Lopez-Ribot JL (2015). In Vitro Activity of Miltefosine against Candida albicans under Planktonic and Biofilm Growth Conditions and In Vivo Efficacy in a Murine Model of Oral Candidiasis. Antimicrob Agents Chemother 59(12): 7611–20.http://dx.doi.org/10.1128/AAC.01890-15
196. Hata K, Horii T, Miyazaki M, Watanabe N-A, Okubo M, Sonoda J, Nakamoto K, Tanaka K, Shirotori S, Murai N, Inoue S, Matsukura M, Abe S, Yoshimatsu K, and Asada M (2011). Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrob Agents Chemother 55(10): 4543–51.http://dx.doi.org/10.1128/AAC.00366-11
197. Groll AH, and Walsh TJ (2006). Antifungal efficacy and pharmacodynamics of posaconazole in experimental models of invasive fungal infections. Mycoses 49(s1): 7–16.http://dx.doi.org/10.1111/j.1439-0507.2006.01296.x
198. Pietrella D, Enjalbert B, Zeidler U, Znaidi S, Rachini A, Vecchiarelli A, and d’Enfert C (2012). A luciferase reporter for gene expression studies and dynamic imaging of superficial Candida albicans infections. Methods Mol Biol 845: 537–46.http://dx.doi.org/10.1007/978-1-61779-539-8_39
199. Ci T, Yuan L, Bao X, Hou Y, Wu H, Sun H, Cao D, and Ke X (2018). Development and anti-Candida evaluation of the vaginal delivery system of amphotericin B nanosuspension-loaded thermogel. . J Drug Target 5: 1–11.http://dx.doi.org/10.1080/1061186X.2018.1434660
200. Peters BM, Luna-Tapia A, Tournu H, Rybak JM, Rogers PD, and Palmer GE (2017). An Azole-Tolerant Endosomal Trafficking Mutant of Candida albicans Is Susceptible to Azole Treatment in a Mouse Model of Vaginal Candidiasis. Antimicrob Agents Chemother 61(6): e00084-17.http://dx.doi.org/10.1128/AAC.00084-17
201. Gao M, Wang H, and Zhu L (2016). Quercetin Assists Fluconazole to Inhibit Biofilm Formations of Fluconazole-Resistant Candida Albicans In Vitro and In Vivo Antifungal Managements of Vulvovaginal Candidiasis. Cell Physiol Biochem 40(3–4): 727–742.http://dx.doi.org/10.1159/000453134
202. Bozó A, Domán M, Majoros L, Kardos G, Varga I, and Kovács R (2016). The in vitro and in vivo efficacy of fluconazole in combination with farnesol against Candida albicans isolates using a murine vulvovaginitis model. J Microbiol 54(11): 753–760.http://dx.doi.org/10.1007/s12275-016-6298-y
203. Andes D, Nett J, Oschel P, Albrecht R, Marchillo K, and Pitula A (2004). Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect Immun 72(10): 6023–31.http://dx.doi.org/10.1128/IAI.72.10.6023-6031.2004
204. Nett JE, Marchillo K, Spiegel CA, and Andes DR (2010). Development and validation of an in vivo Candida albicans biofilm denture model. Infect Immun 78(9): 3650–9.http://dx.doi.org/10.1128/IAI.00480-10
205. Vande Velde G, Kucharíková S, Schrevens S, Himmelreich U, and Van Dijck P (2014). Towards non-invasive monitoring of pathogen-host interactions during Candida albicans biofilm formation using in vivo bioluminescence. Cell Microbiol 16(1): 115–30.http://dx.doi.org/10.1111/cmi.12184
206. Wu H, Liu S, Wiradharma N, Ong ZY, Li Y, Yang YY, and Ying JY (2017). Short Synthetic α-Helical-Forming Peptide Amphiphiles for Fungal Keratitis Treatment In Vivo. Adv Healthc Mater 6(6): 1600777.http://dx.doi.org/10.1002/adhm.201600777
207. Silva-Dias A, Miranda IM, Branco J, Cobrado L, Monteiro-Soares M, Pina-Vaz C, and Rodrigues AG (2015). In vitro antifungal activity and in vivo antibiofilm activity of cerium nitrate against Candida species. J Antimicrob Chemother 70(4): 1083–93.http://dx.doi.org/10.1093/jac/dku511
208. Lazzell AL, Chaturvedi AK, Pierce CG, Prasad D, Uppuluri P, and Lopez-Ribot JL (2009). Treatment and prevention of Candida albicans biofilms with caspofungin in a novel central venous catheter murine model of candidiasis. J Antimicrob Chemother 64(3): 567–70.http://dx.doi.org/10.1093/jac/dkp242
209. Negri CE, Johnson A, McEntee L, Box H, Whalley S, Schwartz JA, Ramos-Martín V, Livermore J, Kolamunnage-Dona R, Colombo AL, and Hope WW (2018). Pharmacodynamics of the Novel Antifungal Agent F901318 for Acute Sinopulmonary Aspergillosis Caused by Aspergillus flavus. J Infect Dis 217(7): 1118–1127.http://dx.doi.org/10.1093/infdis/jix479
210. Colley T, Sehra G, Chowdhary A, Alanio A, Kelly SL, Kizawa Y, Armstrong-James D, Fisher MC, Warrilow AGS, Parker JE, Kelly DE, Kimura G, Nishimoto Y, Sunose M, Onions S, Crepin D, Lagasse F, Crittall M, Shannon J, McConville M, King-Underwood J, Naylor A, Bretagne S, Murray J, Ito K, Strong P, and Rapeport G (2018). In VitroandIn VivoEfficacy of a Novel and Long Acting Fungicidal Azole, PC1244 onAspergillus fumigatusInfection. Antimicrob Agents Chemother AAC.01941-17.http://dx.doi.org/10.1128/AAC.01941-17
211. Kimura G, Nakaoki T, Colley T, Rapeport G, Strong P, Ito K, and Kizawa Y (2017). In Vivo Biomarker Analysis of the Effects of Intranasally Dosed PC945, a Novel Antifungal Triazole, on Aspergillus fumigatus Infection in Immunocompromised Mice. Antimicrob Agents Chemother 61(9): e00124-17.http://dx.doi.org/10.1128/AAC.00124-17
212. Seyedmousavi S, Mouton JW, Melchers WJG, and Verweij PE (2017). In VivoEfficacy of Liposomal Amphotericin B against Wild-Type and Azole-Resistant Aspergillus fumigatus Isolates in Two Different Immunosuppression Models of Invasive Aspergillosis. Antimicrob Agents Chemother 61(6): e02479-16.http://dx.doi.org/10.1128/AAC.02479-16
213. Kai H, Yamashita M, Nakamura I, Yoshikawa K, Nitta K, Watanabe M, Inamura N, and Fujie A (2013). Synergistic antifungal activity of KB425796-C in combination with micafungin against Aspergillus fumigatus and its efficacy in murine infection models. J Antibiot 66(8): 479–484.http://dx.doi.org/10.1038/ja.2013.57
214. Nixon GL, McEntee L, Johnson A, Farrington N, Whalley S, Livermore J, Natal C, Washbourn G, Bibby J, Berry N, Lestner J, Truong M, Owen A, Lalloo D, Charles I, and Hope W (2018). Pharmacodynamics of Flubendazole for Cryptococcal Meningoencephalitis: Repurposing and Reformulation of an Anti-Parasitic Agent for a Neglected Fungal Disease. Antimicrob Agents Chemother AAC.01909-17.http://dx.doi.org/10.1128/AAC.01909-17
215. Santos JRA, Ribeiro NQ, Bastos RW, Holanda RA, Silva LC, Queiroz ER, and Santos DA (2017). High-dose fluconazole in combination with amphotericin B is more efficient than monotherapy in murine model of cryptococcosis. Sci Rep 7(1): 4661.http://dx.doi.org/10.1038/s41598-017-04588-7
216. Jandú JJ, Costa MC, Santos JRA, Andrade FM, Magalhães TF, Silva M V, Castro MCAB, Coelho LCBB, Gomes AG, Paixão TA, Santos DA, and Correia MTS (2017). Treatment with pCramoll Alone and in Combination with Fluconazole Provides Therapeutic Benefits inC. gattiiInfected Mice. Front Cell Infect Microbiol 7: 211.http://dx.doi.org/10.3389/fcimb.2017.00211
217. Nishikawa H, Fukuda Y, Mitsuyama J, Tashiro M, Tanaka A, Takazono T, Saijo T, Yamamoto K, Nakamura S, Imamura Y, Miyazaki T, Kakeya H, Yamamoto Y, Yanagihara K, Mukae H, Kohno S, and Izumikawa K (2017). In vitro and in vivo antifungal activities of T-2307, a novel arylamidine, against Cryptococcus gattii: an emerging fungal pathogen. J Antimicrob Chemother 72(6): 1709–1713.http://dx.doi.org/10.1093/jac/dkx020
218. Wiederhold NP, Shubitz LF, Najvar LK, Jaramillo R, Olivo M, Catano G, Trinh HT, Yates CM, Schotzinger RJ, Garvey EP, and Patterson TF (2018). The Novel Fungal Cyp51 Inhibitor VT-1598 is Efficacious in Experimental Models of Central Nervous System Coccidioidomycosis Caused by Coccidioides posadasii and Coccidioides immitis. Antimicrob Agents Chemother AAC.02258-17.http://dx.doi.org/10.1128/AAC.02258-17
219. López-García B, Lee PHA, Yamasaki K, and Gallo RL (2005). Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol 125(1): 108–15.http://dx.doi.org/10.1111/j.0022-202X.2005.23713.x
220. Ben-Ami R, Lewis RE, Leventakos K, Latgé J-P, and Kontoyiannis DP (2010). Cutaneous model of invasive aspergillosis. Antimicrob Agents Chemother 54(10): 4474–5.http://dx.doi.org/10.1128/AAC.01504-09
221. Kucharíková S, Tournu H, Holtappels M, Van Dijck P, and Lagrou K (2010). In vivo efficacy of anidulafungin against mature Candida albicans biofilms in a novel rat model of catheter-associated Candidiasis. Antimicrob Agents Chemother 54(5): 1848–54.http://dx.doi.org/10.1128/AAC.00697-10
222. Bink A, Kucharíková S, Neirinck B, Vleugels J, Van Dijck P, Cammue BPA, and Thevissen K (2012). The nonsteroidal antiinflammatory drug diclofenac potentiates the in vivo activity of caspofungin against Candida albicans biofilms. J Infect Dis 206(11): 1790–7.http://dx.doi.org/10.1093/infdis/jis594
223. Kucharíková S, Sharma N, Spriet I, Maertens J, Van Dijck P, and Lagrou K (2013). Activities of systemically administered echinocandins against in vivo mature Candida albicans biofilms developed in a rat subcutaneous model. Antimicrob Agents Chemother 57(5): 2365–8.http://dx.doi.org/10.1128/AAC.02288-12
224. Kucharíková S, Neirinck B, Sharma N, Vleugels J, Lagrou K, and Van Dijck P (2015). In vivo Candida glabrata biofilm development on foreign bodies in a rat subcutaneous model. J Antimicrob Chemother 70(3): 846–56.http://dx.doi.org/10.1093/jac/dku447
225. Holtappels M, Swinnen E, De Groef L, Wuyts J, Moons L, Lagrou K, Van Dijck P, and Kucharíková S (2018). Antifungal Activity of Oleylphosphocholine onIn VitroandIn Vivo Candida albicansBiofilms. J Antimicrob Chemother 62(1): e01767-17.http://dx.doi.org/10.1128/AAC.01767-17
226. Li D-D, Zhao L-X, Mylonakis E, Hu G-H, Zou Y, Huang T-K, Yan L, Wang Y, and Jiang Y-Y (2014). In vitro and in vivo activities of pterostilbene against Candida albicans biofilms. Antimicrob Agents Chemother 58(4): 2344–55.http://dx.doi.org/10.1128/AAC.01583-13
227. Maiolo EM, Oliva A, Furustrand Tafin U, Perrotet N, Borens O, and Trampuz A (2016). Antifungal activity against planktonic and biofilm Candida albicans in an experimental model of foreign-body infection. J Infect 72(3): 386–92.http://dx.doi.org/10.1016/j.jinf.2015.12.008
228. Zhao J, Cheng Y, Song X, Wang C, Su G, and Liu Z (2015). A Comparative Treatment Study of Intravitreal Voriconazole and Liposomal Amphotericin B in an Aspergillus fumigatus Endophthalmitis Model. Invest Ophthalmol Vis Sci 56(12): 7369–76.http://dx.doi.org/10.1167/iovs.15-17266
229. Wiederhold NP, Najvar LK, Matsumoto S, Bocanegra RA, Herrera ML, Wickes BL, Kirkpatrick WR, and Patterson TF (2015). Efficacy of the investigational echinocandin ASP9726 in a guinea pig model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 59(5): 2875–81.http://dx.doi.org/10.1128/AAC.04857-14
230. Kirkpatrick WR, Najvar LK, Bocanegra R, Patterson TF, and Graybill JR (2007). New Guinea Pig Model of Cryptococcal Meningitis. Antimicrob Agents Chemother 51(8): 3011–3013.http://dx.doi.org/10.1128/AAC.00085-07
231. Garvey EP, Hoekstra WJ, Schotzinger RJ, Sobel JD, Lilly EA, and Fidel PL (2015). Efficacy of the clinical agent VT-1161 against fluconazole-sensitive and -resistant Candida albicans in a murine model of vaginal candidiasis. Antimicrob Agents Chemother 59(9): 5567–73.http://dx.doi.org/10.1128/AAC.00185-15