Research Articles:
Microbial Cell, Vol. 8, No. 9, pp. 223 - 238; doi: 10.15698/mic2021.09.760
Landscapes and bacterial signatures of mucosa-associated intestinal microbiota in Chilean and Spanish patients with inflammatory bowel disease
1 Programa de Microbiología y Micología, Instituto de Ciencias Biomédicas, Facultad de Medicina, Universidad de Chile, Chile.
2 Programa de Inmunología, Instituto de Ciencias Biomédicas, Facultad de Medicina, Universidad de Chile, Chile.
3 Facultad de Medicina, Departamento de Pediatría y Cirugía Infantil, Campus Oriente-Hospital Dr. Luis Calvo Mackenna, Universidad de Chile, Chile.
4 Facultad de Ciencias Básicas, Departamento de Física, Universidad Metropolitana de Ciencias de la Educación, Santiago, Chile.
5 Programa Enfermedad Inflamatoria Intestinal. Servicio de Gastroenterología, Clínica Las Condes, Santiago, Chile.
6 Gastroenterología, Clínica Universidad de Los Andes, Santiago, Chile.
7 Microbiota-Host Interactions and Clostridia Research Group, Departamento de Ciencias Biológicas, Facultad de Ciencias de la Vida, Universidad Andrés Bello, Santiago, Chile.
8 ANID – Millennium Science Initiative Program – Millennium Nucleus in the Biology of Intestinal Microbiota, Santiago, Chile.
9 Department of Biology, Texas A&M University, College Station, TX, 77843, USA.
10 Department of Gastroenterology and Palma Health Research Institute, Hospital Universitario Son Espases, Palma de Mallorca, Spain.
11 Marine Microbiology Group, Department of Animal and Microbial Diversity, IMEDEA (CSIC-UIB), 07190 Esporles, Illes Balears, Spain.
12 Instituto Milenio de Inmunología e Inmunoterapia, Facultad de Medicina, Universidad de Chile, Chile.
# These authors contributed equally.
Keywords: Mucosa-associated intestinal microbiota, inflammatory bowel disease, ulcerative Colitis, Crohn's Disease, microbiome, bacterial biomarkers, dysbiosis.
Abbreviations:
AUC – area under curve,
CD – Crohn’s disease,
IBD – inflammatory bowel disease,
OPU – operational phylogenetic unit,
OTU – operational taxonomic unit,
UC – ulcerative colitis.
Received originally: 04/01/2021 Received in revised form: 02/06/2021
Accepted: 13/06/2021
Published: 18/06/2021
Correspondence:
Roberto Vidal, Programa de Microbiología y Micología, Facultad de Medicina, Universidad de Chile. Av. Independencia 1027, Independencia, Santiago, Chile. Postal code 8380453; rvidal@uchile.cl
Conflict of interest statement: Funding institutions did not influence the study design or interpretation of the result. Moreover, the authors de-clare that they have no competing interests.
Please cite this article as: Nayaret Chamorro, David A. Montero, Pablo Gallardo, Mauricio Farfán, Mauricio Contreras, Marjorie De la Fuente, Karen Dubois, Marcela A. Hermoso, Rodrigo Quera, Marjorie Pizarro-Guajardo, Daniel Paredes-Sabja, Daniel Ginard, Ramon Rosselló-Móra and Roberto Vidal (2021). Landscapes and bacterial signa-tures of mucosa-associated intestinal microbiota in Chilean and Spanish patients with inflammatory bowel disease. Microbial Cell 8(9): 223-238. doi: 10.15698/mic2021.09.760
Abstract
Inflammatory bowel diseases (IBDs), which include ulcerative colitis (UC) and Crohn’s disease (CD), cause chronic inflammation of the gut, affecting millions of people worldwide. IBDs have been frequently associated with an alteration of the gut microbiota, termed dysbiosis, which is generally characterized by an increase in abundance of Proteobacteria such as Escherichia coli, and a decrease in abundance of Firmicutes such as Faecalibacterium prausnitzii (an indicator of a healthy colonic microbiota). The mechanisms behind the development of IBDs and dysbiosis are incompletely understood. Using samples from colonic biopsies, we studied the mucosa-associated intestinal microbiota in Chilean and Spanish patients with IBD. In agreement with previous studies, microbiome comparison between IBD patients and non-IBD controls indicated that dysbiosis in these patients is characterized by an increase of pro-inflammatory bacteria (mostly Proteobacteria) and a decrease of commensal beneficial bacteria (mostly Firmicutes). Notably, bacteria typically residing on the mucosa of healthy individuals were mostly obligate anaerobes, whereas in the inflamed mucosa an increase of facultative anaerobe and aerobic bacteria was observed. We also identify potential co-occurring and mutually exclusive interactions between bacteria associated with the healthy and inflamed mucosa, which appear to be determined by the oxygen availability and the type of respiration. Finally, we identified a panel of bacterial biomarkers that allow the discrimination between eubiosis from dysbiosis with a high diagnostic performance (96% accurately), which could be used for the development of non-invasive diagnostic methods. Thus, this study is a step forward towards understanding the landscapes and alterations of mucosa-associated intestinal microbiota in patients with IBDs.
INTRODUCTION
The intestinal microbiota plays a key role in human health, providing important metabolic functions, stimulating the immune system, acting as a barrier for pathogenic organisms, and regulating body composition [1][2].
–
Microorganisms colonize the mammalian intestine immediately after birth. In humans, the adult-like configuration of the gut microbial composition is established over the first three years of life [3]. However, the gut microbiota remains largely stable thereafter [4][5], although it is influenced by various environmental factors, including diet, lifestyle, and medication [6]. Moreover, a study conducted on human subjects with a wide age range, specifically 0–104 years, showed that the gut microbiota composition changes sequentially with age and that nutrients may play a key role in such changes [7]. Therefore, changes in microbiota structure or dysbiosis have been associated with alterations in diet [8], in addition to chronic stress [9], antibiotic use and a number of gastrointestinal disorders [10][11][12][13][14][15], such as inflammatory bowel disease (IBD) [16][17].
–
Within IBD phenotypes, ulcerative colitis (UC) and Crohn’s disease (CD) have a clinical impact worldwide, with UC characterized by inflammation of the rectum, extending diffusely towards the colon, whereas CD is characterized by systemic inflammation and ulcers affecting any part of the gastrointestinal tract and thickening of the intestinal wall. Since 1990, the incidence of IBD has increased in Africa, Asia and South America [18]; although no data are available in Chile, clinical experience shows an increase in recent years in the number of consultations by IBD patients [19][20].
–
While the etiological causes of UC and CD remain unidentified, several factors are known to increase susceptibility, including: i) genetic polymorphisms [21][22][23]; ii) altered immune response [24][25] iii) environmental factors [26][27][28]; and (iv) alteration of the gut microbiota composition [29][30][31][32][33][34].
–
In general, studies of the gut microbial composition of patients with UC and CD have shown an increase in the phylum Proteobacteria and a decrease in the phylum Firmicutes [35][36]. Most of these studies have mainly analyzed stool samples. However, many differences between the microbial communities from fecal and mucosal samples have been reported, indicating that fecal microbial communities do not accurately represent the local communities that live in specific regions of the gut (colon, ileus and small intestine) [25][37][38][39]. In addition, most of these studies are based on the massive sequencing of amplicons of the 16S rRNA gene and the clustering of reads into operational taxonomic units (OTUs), using short sequences of variable regions of the gene [40][41]. The short size of the amplicons (<300 nt) only allows the identification up to the family level or in some cases genus.
–
In the present study, we investigated the mucosa-associated intestinal microbiota in Chilean and Spanish patients with IBD. For this, we amplified the 16S rRNA gene and obtained sequence libraries from colonic mucosa biopsies using high-quality reads of more than 300 nucleotides for the bacterial affiliation process. In addition, we used the OPU (Operational Phylogenetic Unit) approach for taxonomic assignment, as this allows for better bacterial identification, in most cases reaching the species level [42]. OPU analysis is not based on strict identity thresholds; instead, sequences are affiliated with a phylogenetic tree using the parsimony algorithm followed by manual supervision of the tree to design meaningful phylogenetic units [43]. Since identification is based on phylogenetic inference, OPUs are based on the genealogical signal of the sequences, which minimizes the influence of errors and size differences. An OPU is the smallest monophyletic group of sequences containing OTU representatives together with the closest reference sequence, including the sequence of a type strain when possible [44]. To date, there have been no reports on the microbiome of patients with UC at the OPU level.
–
Our results indicate that some IBD patients have an intestinal dysbiosis, while some others showed a microbiota profile similar to that of control individuals. In dysbiotic patients, we found an increase in pro-inflammatory bacteria (mostly Proteobacteria) and a decrease in beneficial commensal bacteria (mostly Firmicutes). We also identified potential co-occurring and mutually exclusive interactions between bacteria associated with the healthy and inflamed mucosa, which appear to be determined by oxygen availability and the type of respiration. Importantly, these results were consistent with the “Oxygen Hypothesis”, which states that chronic inflammation induces increased oxygen levels in the gut, leading to an imbalance between obligate and facultative anaerobes [33]. Finally, we identified bacterial biomarkers that could be used for the development of non-invasive diagnostic methods, such as real-time PCR. A future goal is that an early detection of changes in the gut microbiota could allow the initiation of IBD treatment or preventive measures.
RESULTS
Here we focused on two independent cohorts of patients with IBD from Chile and Spain. Both cohorts were adults and included patients diagnosed with UC or CD. In addition, control individuals (CTL; Non-IBD controls) who underwent colonoscopy due to a family history of colon cancer were included. All samples were colonic mucosal biopsies. Patients who received antibiotic treatment within one month prior to the colonoscopy were excluded. The Chilean cohort included 20 and 21 patients with UC and CD, respectively, and five control individuals. The Spanish cohort was previously reported by Vidal et al., 2015 [42], of which we included 13 patients with CD and also seven control individuals. The clinical features of the patients are described in Table S1. For both cohorts, microbial composition in colonic biopsies were assessed by DNA extraction followed by 16S rRNA gene pyrosequencing, as described in the Materials and Methods section.
–
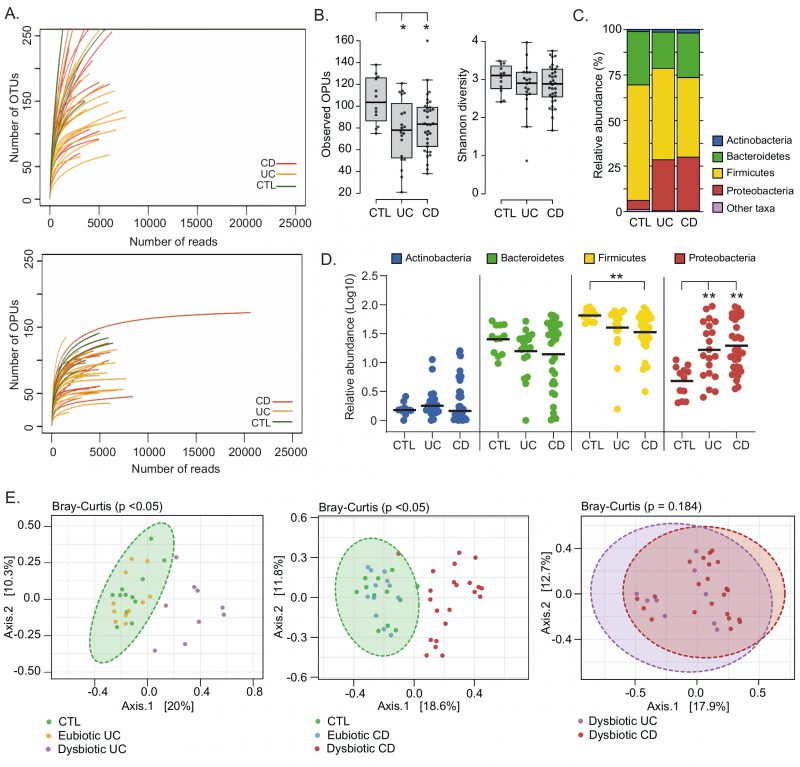
Pyrosequencing of Chilean samples generated 331,677 reads, which together with the Spanish samples generated a total of 483,818 reads with a mean of 5,506 reads per sample (Table S2). The average read length for 16S rRNA sequences was 649 bp. The OTUs from Chilean samples were clustered and inserted into the tree by Vidal et al., 2015 [42]. OTUs that were not clustered as part of known OPUs were re-evaluated and designated anew. This resulted in a total of 608 OPUs, with a mean of 88 OPUs per sample (Table S2). Notably, our results show that the OPU rarefaction curves approached saturation with a significantly lower number of reads than necessary for the OTU curves, indicating an overestimation of taxonomic units (diversity) when using a traditional OTU approach (Fig. 1A).
–
–
Gut microbial dysbiosis among IBD patients
Differences in the gut microbiota of IBD patients compared to healthy individuals have been widely reported [45][46][47][48]. Among IBD patients there may be several degrees and profiles of dysbiosis, which have been correlated with the phenotype and severity of the disease [40][42][49], and gastrointestinal surgery [50][51]. Therefore, we first assessed the landscape, richness, and diversity of the mucosa-associated intestinal microbiota among patients and non-IBD controls. In general, we found that UC and CD patients have a significantly lower richness (Observed OPUs) than the controls. Despite, no significant differences in the alpha diversity (Shannon diversity) were observed (Fig. 1B). IBD patients studied for a long time in relation to the composition of their microbiota in biopsies showed the existence of temporary structure changes, even with microbiota profiles similar to healthy controls, probably associated with treatment [46] and to the remission of the disease [47]. Therefore, we cannot rule out that a similar alpha diversity between some patients and control individuals in our work could be a consequence of treatments and/or disease remission at the time the biopsies samples were taken.
–
Four major bacterial phyla (Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria) dominate the gut microbiota of humans [38]. Accordingly, almost all OPUs identified were affiliated with one of these phyla. In general, we observed that UC and CD patients had a lower abundance of Firmicutes and a higher abundance of Proteobacteria (Fig. 1C and 1D). Interestingly, a principal coordinate analysis (PCoA) based on the Bray-Curtis dissimilarity showed that only some patients had a significant shift in beta diversity (dysbiosis) compared to the controls (Fig. 1E). This result suggests that some patients (dysbiotic UC and CD patients) but not all (eubiotic UC and CD patients) had an alteration in their mucosa-associated intestinal microbiota. Of note, there were no significant differences in beta diversity between dysbiotic patients that clustered away from control individuals, suggesting they had a similar imbalance in their microbiota.
–
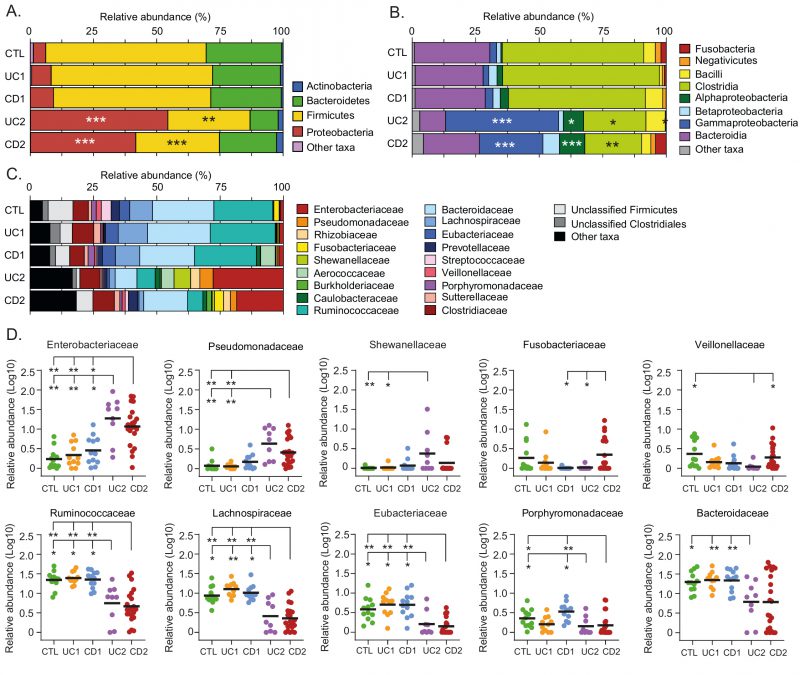
Based on the PCoA analysis and the IBD phenotype, patients were subdivided into four groups: UC1, UC2, CD1 and CD2 (Table S1). Consistent with this subclassification, hierarchical clustering using the relative abundances of the 608 OPUs across all samples showed that UC1, CD1 and controls clustered together but away from UC2 and CD2 (Fig. 2A). Moreover, relative abundances per patient at the phylum level clearly showed that while UC1, CD1 and controls had a similar taxonomic composition, UC2 and CD2 had a dysbiosis characterized by an increase in Proteobacteria and a decrease in Firmicutes (Fig. 2B). As expected, UC1 and CD1 showed a richness and diversity similar to the controls. By contrast, UC2 and CD2 had significantly lower richness than the controls, but only CD2 showed a lower alpha diversity (Fig. 2C). Thus, collectively these results indicate variability in the mucosa-associated intestinal microbiota among IBD patients: while some patients have a dysbiotic microbiota, others have a microbiota composition similar to that of the non-IBD controls.
–
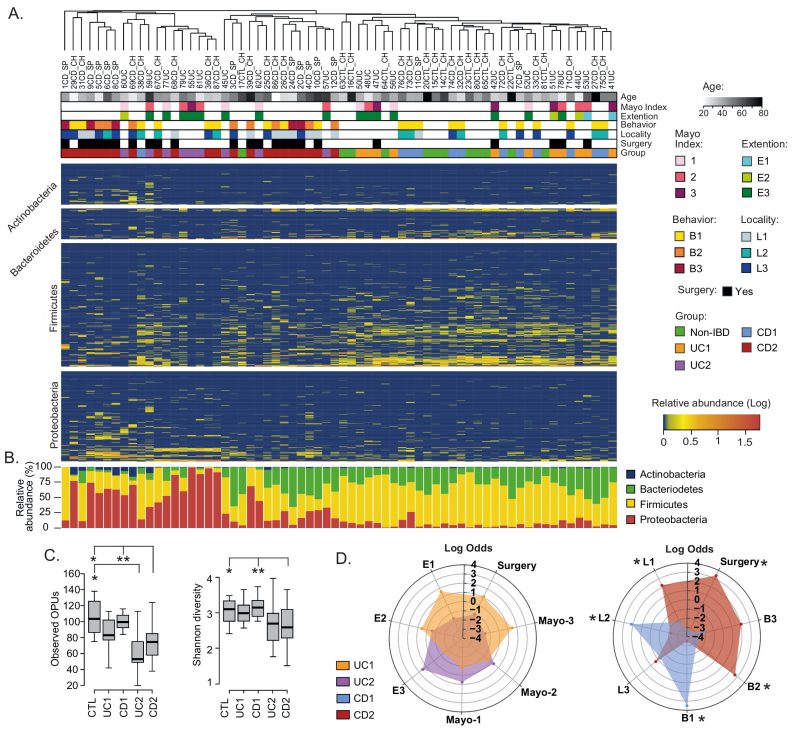 | FIGURE 2: Compositional differences in the mucosa-associated intestinal microbiota among IBD patients.(A) Clustering of patients and controls and heatmap of OPU abundances. Patients (Columns) were grouped based on a hierarchical cluster analysis using relative abundances of 608 OPUs. OPUs of the four most abundant phyla are shown in the rows, and their relative abundances are shown on a log scale according to the legend. The clinical and demographic features of the patients are shown at the top and are colored according to the legend. Note that in general, patients belonging to the UC2 and CD2 groups clustered together and away from patients in the UC1 and CD1 groups and the controls (Non-IBD). (B) Relative abundances (%) per patient of the four most abundant phyla. (C) Alpha diversity between IBD groups (UC1, UC2. CD1, and CD2) and control individuals determined by the observed OPUs (Richness) and the Shannon index (Diversity). Significance: * p< 0.05, ** p< 0.005, Kruskal-Wallis & Dunn's tests. (D) Radar chart showing association of IBD groups with clinical features. Pairwise association between patient groups and clinical features was performed in contingency tables by odds ratios. Significance: * p< 0.05, Pearson's chi-squared test or Fisher's exact test. The figure was prepared using the Plotly package [52] in R [53]. |
–
Dysbiosis in Crohn’s patients is correlated with disease severity
We then asked whether patient groups correlated with demographic and clinical variables. Regarding demographic features, no significant correlations were found with respect to age, sex, or origin of the patients (not shown). Similarly, there were no significant correlations of the UC1 and UC2 groups with clinical variables. By contrast, while the CD1 group correlated with colonic location (L2) and an inflammatory disease behavior (B1), the CD2 group correlated with ileal location (L1), the stricturing (B2) and penetrating (B3) phenotypes, and surgery (Fig. 2D). Thus, patients in the CD2 group were associated with an advanced stage of the disease, which suggests a clinically relevant link between gut microbiota dysbiosis and disease severity.
–
Dysbiosis in IBD is characterized by an increase in pathobionts and a decrease in anti-inflammatory commensal bacteria
Although it is not known whether dysbiosis is a cause or a consequence of IBD, the identification of specific taxa and bacteria (biomarkers) associated with these patients may contribute to understand the dynamics of this disease (progression or remission), as well as the development of diagnostic aids [54]. Therefore, we sought to characterize the variation in the gut microbiota at different taxonomic levels among the IBD patients and controls. We found that the relative abundances at the phylum and class levels were similar between the UC1, CD1 and control groups, further confirming that these IBD patients had a non-dysbiotic gut microbiota (eubiotic status; Fig. 3). Conversely, in the UC2 and CD2 groups, the phylum Firmicutes was decreased, mainly attributed to a lower abundance in Clostridia, while the phylum Proteobacteria was increased, mainly attributed to a higher abundance in Alpha- and Gammaproteobacteria (Fig. 3A and 3B).
–
–
At the family level, we found that the UC2 and CD2 groups had a greater abundance of Enterobacteriaceae and Pseudomonadaceae. Additionally, Shewanellaceae was increased in UC2. The shift in the gut microbiota composition in the UC2 and CD2 groups was also characterized by a decreased abundance of Ruminococcaceae, Lachnosporiraceae, Eubacteriaceae, Porphyromonadaceae and Bacteroidaceae (Fig. 3C and 3D). On the other hand, differences between the UC2 and CD2 groups were only observed in Fusobacteriaceae and Veillonellaceae, with both taxa being decreased in the UC2 group. Hence, the dysbiotic profile of UC2 and CD2 patients was in general very similar.
–
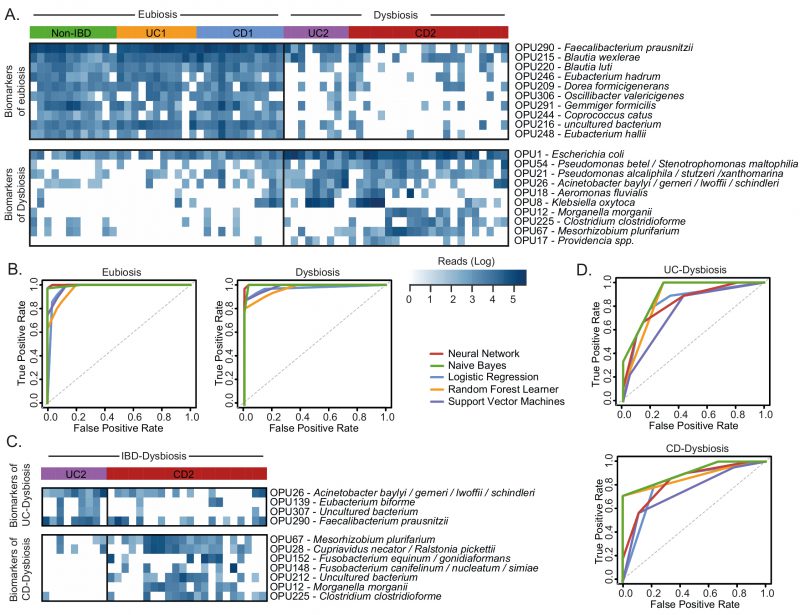
To further investigate the microbial imbalance in the IBD, we used the LEfSe algorithm to identify which OPUs were differentially abundant in the dysbiotic patients (UC2 and CD2 groups) compared to the eubiotic patients (UC1 and CD1 groups) and controls. As a result, 24 OPUs were found to be significantly increased in dysbiotic patients, including several potential pathogens (pathobionts) such as Escherichia coli, Klebsiella oxytoca, Ruminococcus gnavus, Enterococcus faecalis, several Rhizobium, Pseudomonas and Clostridium species, and members of the tribe Proteeae (Providencia/Morganella) (Fig. 4A, Table S3). By contrast, 25 OPUs were found to be decreased, including several symbionts known to provide important functions for gut health, mainly through the production of butyrate [55][56]. For instance, butyrate producers such as Faecalibacterium prausnitzii, Blautia, Gemmiger formicilis, Eubacterium rectale, Ruminococcus torques, Roseburia inulinivorans and Coprococcus catus were significantly decreased. Other commensal bacteria such as Alistipes and Dorea were also decreased in these patients. Thus, collectively these results indicate that the dysbiosis in these IBD patients is characterized by an increase in pathobionts and a decrease in beneficial commensal bacteria.
–
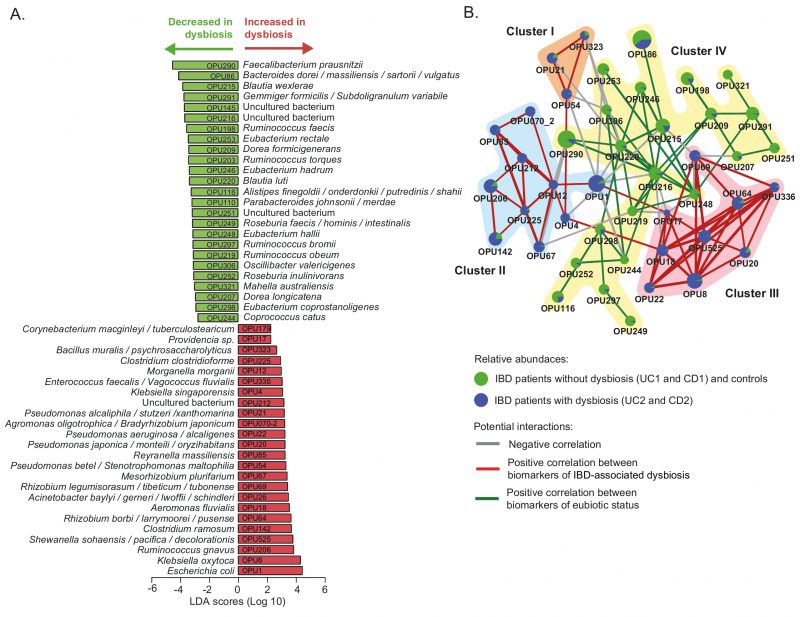 | FIGURE 4: Differently abundant OPUs in dysbiotic IBD patients and their co-existence networks.(A) The LEfSe algorithm with a linear discriminant analysis (LDA) score >2 enabled the identification of OPUs that differed significantly in abundance between the dysbiotic patients (UC2 and CD2 groups) and eubiotic patients (UC1 and CD1) and non-IBD controls (B) Correlation networks of differently abundant OPUs. This analysis was performed using SparCC [57] (see methods). The nodes (pie charts) represent OPUs, with size reflecting the relative abundance in IBD patients and controls, as described in the legend. The links between nodes correspond to significant interactions (positive or negative correlations), with line width reflecting the strength of the correlation. Clusters (I to IV) of highly correlated OPUs are indicated. Unconnected nodes were omitted. |
–
Pathobionts increased in dysbiotic IBD patients have co-occurring relationships and do not co-exist with the core bacteria of the eubiotic state
In the context of IBD, unraveling potential interactions between bacteria associated with a healthy or an affected mucosa can contribute to understand how the microbiota responds and adapts to an inflammatory environment. The correlation network analysis has been used to predictively model the interplay between the microbiota and the environment [58]. Consequently, in the following we explored the potential interactions among the OPUs that were differentially abundant in IBD patients using the SparCC algorithm [57].
–
Notably, this analysis revealed that pathobionts increased in dysbiosis form three clusters (I, II and III) of positive co-occurring relationships (Fig 4B). In particular, Cluster I (OPU21, OPU54 and OPU323) and Cluster III (OPU8, OPU17, OPU18, OPU20, OPU22, OPU64, OPU69, OPU336 and OPU525) include facultative anaerobic and aerobic bacteria, and Cluster II (OPU1, OPU4, OPU12, OPU67, OPU70-2, OPU85, OPU142, OPU206, OPU212 and OPU225) includes obligate anaerobic, facultative anaerobic and aerobic bacteria (Table S3). Furthermore, these three clusters have negative correlations (mutual exclusivity) with Cluster IV, which is formed by obligate anaerobic bacteria that were significantly abundant in patients without dysbiosis and the controls. Overall, this result indicates that bacteria of Clusters I and III and the majority of Cluster II can survive in a niche with a high level of oxygen. Therefore, oxygen levels at the intestinal mucosa could be an environmental characteristic that determines the type of relationship (co-existence or mutual exclusivity) between these bacteria.
–
Dysbiosis in IBD patients is characterized by a shift from obligate to facultative anaerobes and to aerobic bacteria
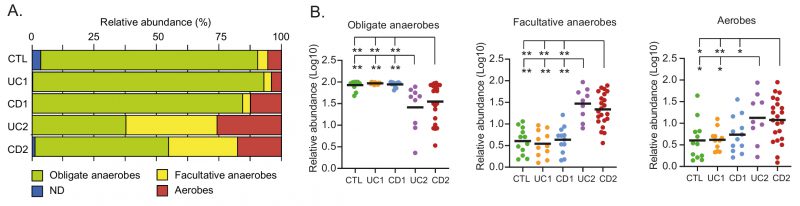
The healthy intestinal mucosa has low levels of oxygen and thereby allows the survival and establishment of bacterial communities of obligate anaerobes. By contrast, dysbiosis in IBD is characterized by an increase in facultative anaerobes from the phylum Proteobacteria. Furthermore, it has been hypothesized (Oxygen Hypothesis) that chronic inflammation leads to an increased oxygen levels in the gut, which in turn creates a microenvironment that favors facultative anaerobes or even aerobic bacteria [33]. In light of these observations, we evaluated the oxygen hypothesis by investigating the microbial respiration of mucosa-associated intestinal microbiota among the IBD patients. Interestingly, consistent with the oxygen hypothesis and previous studies [59][60], we found that the IBD patients with dysbiosis (UC2 and CD2) had a decreased abundance of obligate anaerobes and an increased abundance of facultative anaerobes and aerobic bacteria (Fig. 5A-B). Thus, this result, together with the correlation network analysis, strongly suggests a key role for oxygen in the intestinal dysbiosis of IBD patients.
–
–
Bacterial biomarkers make it possible to discriminate dysbiosis and eubiosis in IBD patients and between UC and CD patients with dysbiosis
Currently, IBD has no clear etiology, and due to non-specific symptoms, diagnosis in some patients can be delayed or missed [61]. In this sense, diagnostics is a promising application of the microbiota association studies. It is important to note that instead of classifying IBD patients based on the overall gut microbiota composition, the use of a small set of bacterial biomarkers would be more feasible and economical in clinical practice. Moreover, since dysbiosis was not observed among all IBD patients at the time of sampling, we rationalize that as a first approach, discriminating dysbiosis from eubiosis in these patients could be a valuable diagnostic aid.
–
Therefore, we evaluated the 49 differentially abundant OPUs as biomarkers using the ROC curve analysis. This analysis showed that some of these OPUs could discriminate dysbiosis from eubiosis but with a fair to poor diagnostic performance (Figure S1). The best performance as biomarkers were achieved by F. prausnitzii (OPU290), Blautia luti (OPU220), C. catus (OPU244), Eubacterium hadrum (OPU246) and E. coli (OPU1), with an area under the curve (AUC) ranging from 0.87 to 0.94.
–
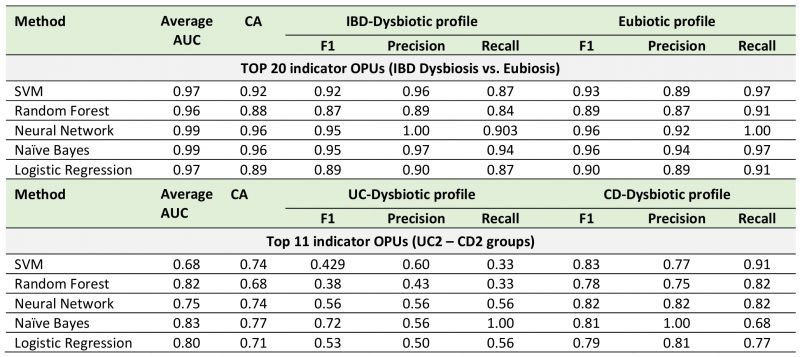
Next, to improve the diagnostic performance achieved by individual biomarkers, we used a combinatorial panel of OPUs and implemented five machine learning classification methods (Neural Network, Naïve Bayes, Logistic regression, Random Forest, and Support Vector Machines; see methods). For this, we selected ten biomarkers for eubiosis and ten biomarkers for dysbiosis based on their AUC. As a result, we found that this panel of 20 biomarkers enabled dysbiosis to be distinguished from eubiosis with a high discriminatory power, with an AUC ranging from 0.96 to 0.99 (Fig. 6, Table 1). In particular, the Neural Network and Naïve Bayes models were the best performing classifiers, both with an overall accuracy of 96% (Table 1).
–
–
Finally, because the microbiota composition between the UC and CD patients with dysbiosis was remarkably similar, we sought to clarify whether bacterial biomarkers can discriminate between these groups of patients. For this, we implemented the same prediction pipeline shown above, that is, the LEfSe algorithm followed by a ROC curve analysis and machine learning classification methods. In this case, we selected a panel of four biomarkers of UC patients with dysbiosis and seven biomarkers for CD patients with dysbiosis (Fig. 6C). By using these eleven biomarkers, the Naïve Bayes model was the best classifier, achieving an AUC and accuracy of 0.83 and 77%, respectively (Fig. 6D, Table 1). Collectively, these results highlight the potential use of these bacterial markers as a diagnostic aid in IBD.
–
TABLE 1. Cross-validation of bacterial biomarkers and machine learning methods for diagnostic aids in IBD. |
 |
DISCUSSION
Several studies have investigated the gut microbiota composition in patients with IBD, but most have mainly analyzed stool samples [47][48][49]. Fecal samples are readily available and easily collected, allowing easy longitudinal sampling within individuals with no major methodological complexities. However, fecal microbial communities do not accurately represent the mucosa-associated microbiota that live in the gastrointestinal tract (e.g. colon, ileus and small intestine) [37][38][39]. Moreover, characterization of the microbiota at mucosal lesion sites provides relevant insights and more accurate conclusions regarding taxonomic composition and dysbiosis in gastrointestinal disorders such as IBD [28][62]. For instance, Gevers et al.[41] investigated the composition of the microbiota in IBD patients using different sample types (stool samples and tissue biopsies of the ileum and rectum) and found that the rectal mucosa-associated microbiota has the potential for an early and accurate diagnosis of CD. By contrast, the com-position of the fecal microbiota was less informative with a poor diagnostic performance in IBD.
–
In this work, we determined the landscapes and bacterial signatures of mucosa-associated microbiota in two independent cohorts of Chilean and Spanish patients diagnosed with IBD. For this, we used the OPU concept for taxonomic assignment [42], which allowed us to reach the species level with a lower number of reads than needed for the traditional OTU-based approach (Fig. 1A). Our results showed that while some IBD patients had an imbalance in the mucosa-associated microbiota, other patients had a microbial composition that was similar to that of non-IBD controls (Fig. 1B-E), probably as a consequence of treatments and/or remission of the disease at the moment of the sampling. Hence, we decided to subdivide the patients (based on the microbiota composition and the IBD phenotype) into four groups: two eubiotic groups (UC1 and CD1) and two dysbiotic groups (UC2 and CD2). Importantly, this subclassification was consistent with the hierarchical clus-tering of patients in which the UC1, CD1 and non-IBD controls clustered together but away from UC2 and CD2 (Fig. 2A). Our results showed that dysbiosis in UC2 and CD2 was characterized by an increase in Proteobacteria and a decrease in Firmicutes (Fig. 2B and Fig. 3). Previous studies have also shown the same alteration in the abundance of these phyla in the mucosa-associated microbiota of IBD patients [35][63][64]. Moreover, differences between the UC2 and CD2 groups were only observed at the family level, with a decreased abundance of Fusobacteriaceae and Veillonellaceae in the UC2 group (Fig. 3D). Despite the similarities in the microbial composition of UC2 and CD2 groups, dysbiosis was correlated with disease severity only in the latter group (Fig. 2D).
–
Several bacteria (pathobionts) that were found to be increased in the mucosal samples of dysbiotic IBD patients are known to have the potential to exacerbate inflammation (Fig. 4A and Table S3). For instance, pathogenic E. coli strains with the ability to adhere and invade the intestinal mucosa have been isolated with a high frequency from biopsies of CD patients [31][32]. K. oxytoca causes antibiotic-associated hemorrhagic colitis [65][66]. Ruminococcus gnavus produces an inflammatory polysaccharide and an increased abundance of this bacterium in IBD patients has been linked to an increase in disease activity [67][68][69]. E. faecalis metalloprotease GelE disrupts the epithelial barrier and increased intestinal inflammation in interleukin-10 knockout mice [70][71][72]. An increased abundance of Rhizobium spp has been shown in CD patients with recurrence after surgery compared to those who remain in remission [51]. Similarly, an increased abundance of Pseudomonas spp has been reported in IBD patients [49][73]. Moreover, Pseudomonas spp. can attach to intestinal epithelial cells and deliver toxins to host cells through a type 3 secretion system, thereby causing epithelial cell damage [74][75]. Clostridium ramosum has been reported to be increased in pediatric patients with CD and induces ulcerative colitis in the DSS-colitis mouse model [76][77].
–
Dysbiosis in IBD patients was also characterized by a decreased abundance of several commensal bacteria with anti-inflammatory properties, including several butyrate producers such as F. prausnitzii, Blautia, G. formicilis, E. rectale, R. torques, R. inulinivorans and C. catus[78]. This result is in agreement with previous studies showing a reduced abundance of multiple butyrate-producing bacteria in dysbiotic IBD patients [46][50][79]. Butyrate is a short-chain fatty acid that plays an important role in maintaining the integrity of colonic mucosa and regulating cell proliferation and differentiation [55][56]. Studies on biopsies from patients with CD cultured with butyrate showed a dose-dependent decrease in the expression of proinflammatory cytokines [80]. In particular, F. prausnitzii is known to be a core bacterium of the gut microbiota of healthy adults, representing around 5% of the total bacterial population. This bacterium plays an important role in butyrate production and has a strong anti-inflammatory effect [78]. Many studies have shown a decreased abundance of this bacterium in IBD patients, this reduction being a bacterial signature of dysbiosis as well as the severity and activity of the disease [29][81][82]. Additional studies are needed to investigate whether the increased abundance of the pathobiont and the depletion of butyrate-producing bacteria may trigger pro-inflammatory signals that contribute to the development or progression of IBD.
–
An interesting finding of this study was the identification of potential co-occurring relationships between pathobionts associated with the inflamed mucosa (Fig. 4B). In turn, these pathobionts showed mutually exclusive relationships with the core bacteria of the healthy mucosa. In an ecological context, a cluster network of highly correlated organisms could be supported by synergistic, related, or shared biological function, including metabolism, respiration, trophic chains, specific bacterial features (immune evasion, aggregation / biofilm formation) and habitat characteristics (pH, salinity, immune recognition, mucins, among others). In line with this idea, we found that most of the OPUs that form Clusters I, II and III are facultative anaerobic or aerobic bacteria. Conversely, the OPUs of Cluster IV are obligate anaerobic bacteria (Table S3). Thus, pathobionts belonging to Clusters I, II and III could be favored by the inflammation and thickness reduction of the mucus layer, which leads to a higher oxygen level in the intestinal epithelium. Moreover, this result is consistent with the study by Hughes et al., 2017 [59], in which formate oxidation and oxygen respiration were identified as metabolic signatures for inflammation-associated dysbiosis.
–
Importantly, we observed an increased abundance of facultative anaerobes and aerobic bacteria in dysbiotic IBD patients (Fig. 5), which is consistent with the oxygen hypothesis. This hypothesis states that chronic inflammation in IBD patients leads to a greater release of hemoglobin (which carries oxygen) and reactive oxygen species to the intestinal lumen. Thus, the increase in oxygen levels causes a disruption in anaerobiosis that confers selective advantages to facultative anaerobes and aerobes, allowing them to become more competitive and able to overgrow [33].
–
Cost-effective, rapid, and reproducible biomarkers would be helpful for patients and clinicians in the diagnosis of IBD. Several bacterial biomarkers for IBD diagnosis have been evaluated in previous studies with promising results [47][61][83][84][85]. The panel of bacterial biomarkers identified in the current study made it possible to discriminate dysbiosis from eubiosis in IBD with a high discriminatory power (96% accurately) (Fig. 6A). Likewise, our bacterial biomarkers discriminated between the dysbiotic UC patient from the dysbiotic CD patients, although with a lower diagnostic performance (77% accurately) (Fig. 6B). The cross-validation of these bacterial biomarkers in different cohorts will confirm their potential use as a diagnostic aid. Finally, these biomarkers could be combined with imaging techniques and calprotectin levels to improve the diagnosis of IBD, thereby facilitating clinical decision-making.
MATERIALS AND METHODS
Patients
Two independent cohorts of patients with IBD from Chile and Spain were included in this study (Table S1). Both cohorts were adults and included 20 Chilean patients diagnosed with ulcerative colitis (UC_CH), 21 Chilean patients diagnosed with Crohn’s disease (CD_CH) and five Chilean non-IBD control individuals (CTL_CH) who underwent a colonoscopy due to a family history of colon cancer. The Spanish cohort was previously reported by Vidal et al., 2015 [42], of which we included 13 patients with CD and seven non-IBD control individuals. Patients who received antibiotic treatment within one month prior to the colonoscopy were excluded.
–
Ethics approval
The study was approved by the Institutional Review Board of Clínica Las Condes, Faculty of Medicine, Universidad de Chile; Ethics Committee of the Northern Metropolitan Health Service, Santiago, Chile; and the Balearic Islands’ Ethics Committee, Spain. Study participants provided written informed consent before entering the study. All records and information were kept confidential and all identifiers were removed prior to analysis.
–
Nucleic acid extraction, 16S rDNA amplification and pyrosequencing
Total DNA was extracted from biopsy samples using the E.Z.N.A. DNA/RNA Isolation kit (Omega-Bio-Tek) following the manufacturer’s recommendations. The rRNA 16S gene was amplified from the extract using the universal primers GM3 (5′-AGAGTTTGATCMTGGC-3′) and 907r (5′-CCGTCAATTCMTTTGAGTTT-3′). A nested PCR with the amplified product was performed using 454 primers. The primer sequences for the nested PCR are listed in Table S4. This purified product was sequenced using the 454 GS-FLX+ platform (Macrogen, Seoul, South Korea). The datasets of Spanish subjects generated during the current study are available in the ENA sequence repository under the project accession numbers PRJEB6107 and ERP005574.
–
Sequence trimming, chimera verification and OTU grouping
Data were processed using a Mothur pipeline [86]. We eliminated low-quality sequences, defined as: sequences under 300 bp, those with a window size and average quality score of 25, a maximum homopolymer of eight nucleotides, without ambiguities and reading mismatches with barcodes primers. Chimeras were eliminated using Chimera Uchime implemented in Mothur. Sequences were grouped into OTUs with 99% [42] identity using the UCLUST package in QIIME [87]. The most abundant reading of each OTU was chosen as its representative.
–
Phylogenetic affiliation and OPU determination.
Representative OTUs, grouped into OPUs in a previous study [42] on samples from Spanish patients, were added to the LTP111 database. These samples were aligned using SINA [88][89] and incorporated into ARB [43] using the maximum parsimony model. The sequences were grouped into OPUs based on a manual inspection of their genealogy. Note that an OPU is the smallest monophyletic group of sequences containing OTU representatives and their closest reference sequence, including a type strain when possible, and is the result of a phylogenetic inference by inserting the new sequences in a preexisting tree using the parsimony tool implemented in the ARB program package [43]. This OPU approach allows combining 16S rRNA gene fragments of distinct length and position within the gene, and therefore obtain comparable results between the amplicon of different samples even with different sequencing strategies. The OPU approach reduces the diversity measures as groups sequences in lineages that approach the species thresholds [42] and therefore gives a more robust view of the microbiome diversity.
–
Compositional, comparative, and statistical analysis
For the statistical analysis and visual exploration, the microbiome data were uploaded to the MicrobiomeAnalyst server [90]. An alpha diversity analysis was calculated based on observed OPUs (Richness) and Shannon Index (Diversity). Beta diversity was calculated based on the principal coordinate analysis (PCoA) using the Bray-Curtis dissimilarity. A hierarchical cluster analysis based on the relative abundances of 608 OPUs was performed using the Bray-Curtis dissimilarity metric and Ward’s linkage. Heat maps showing OPU abundances were drawn using the gplots package [91] in R [53].
–
Statistical differences in the abundance of specific taxa between groups were determined using the Kruskal-Wallis test followed by Dunn’s multiple comparisons test. The variation in the read counts and relative abundances across patients can hamper and bias the identification of disease-associated taxa [45] Indeed, as the taxonomic level decreases, there are fewer taxonomic units and therefore, the subject-to-subject variation can lead to the loss of statistical power. To overcome this problem, logarithmic transformation has been implemented in several microbiome studies, allowing better association analyses compared to the linear scale [45][92]. Consequently, analysis at family level and below were made on data that was transformed using the log(X+1) method.
–
To determine differentially abundant taxa in different groups, a linear discriminant analysis effect size (LEfSe) [93] was performed in the MicrobiomeAnalyst server [90]. For this, a p-value cut off at 0.05 and linear discriminant analysis (LDA) score threshold 2.0 were used. The LEfSe is an algorithm that detects species and functional characteristics that are differentially abundant between two or more environments with statistical significance, effect relevance, and biological consistency. The LEfSe uses the LDA to estimate the effect size of each differentially abundant feature. To explore the potential interactions among OPUs, a correlation network analysis was performed by using the SparCC algorithm [57].
–
Bacterial biomarkers and classifier validation
We defined as biomarkers those OPUs identified by the LEfSe algot which were present in more than half of the samples of the corresponding group (i.e., dysbiosis vs. eubiosis and dysbiotic UC patients vs. dysbiotic CD patients). In order to evaluate the performance of each indicator OPU in discriminating between groups, we first performed a receiver operating characteristic (ROC) curve analysis in the easyROC server [94]. Next, we selected the best discriminating OPUs and implemented several classification algorithms, including Naive Bayes (NB), Random Forest (RF), Logistic Regression (LR) and Support Vector Machine (SVM) and Neural Network (NN) using the Orange data mining suite, V.3.27.0 (http://orange.biolab.si) [95]. NB is a generative model, RF is an ensemble method using decision trees, whereas SVM, LR and NN are discriminative models. We used 70% of samples as the training set, and the rest were used as the test set with the five-fold cross-validation method. The results of the cross-validation (classification accuracy, sensitivity, and specificity) were registered and depicted by ROC curves.
–
This study was supported by Fondo Nacional De Desarrollo Científico y Tecnológico FONDECYT grant 1161161 to R. Vidal, CONICYT-PCHA/2014-21140975 fellowship to N. Chamorro, FONDECYT 1120577 and 1170648 to Hermoso MA and the Spanish Ministry of Economy projects CLG2015 66686-C3-1-P to Rosselló-Mora R., as well as funds from the European Regional Development Fund (FEDER) and NSF Dimensions in Biodiversity grant OCE-1342694. Support was also provided by a Millennium Science Initiative grant from the Ministry of Economy, Development and Tourism to Paredes-Sabja D.
REFERENCES
- Hooper LV, Littman DR, and Macpherson AJ (2012). Interactions between the microbiota and the immune system. Science 336(6086): 1268–1273. 10.1126/science.1223490
- Wang Y, Kuang Z, Yu X, Ruhn KA, Kubo M, and Hooper L V (2017). The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science 357(6354): 912–916. 10.1126/science.aan0677
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, and Gordon JI (2012). Human gut microbiome viewed across age and geography. Nature 486(7402): 222–227. 10.1038/nature11053
- Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, and Gordon JI (2013). The long-term stability of the human gut microbiota. Science 341(6141). 10.1126/science.1237439
- Lim MY, Rho M, Song YM, Lee K, Sung J, and Ko G (2014). Stability of gut enterotypes in Korean monozygotic twins and their association with biomarkers and diet. Sci Rep 4: 1–7. 10.1038/srep07348
- Sommer F, and Bäckhed F (2013). The gut microbiota-masters of host development and physiology. Nat Rev Microbiol 11(4): 227–238. 10.1038/nrmicro2974
- Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, Abe F, and Osawa R (2016). Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol 16(1): 1–12. 10.1186/s12866-016-0708-5
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling A V., Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, and Turnbaugh PJ (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505(7484): 559–563. 10.1038/nature12820
- Gao X, Cao Q, Cheng Y, Zhao D, Wang Z, Yang H, Wu Q, You L, Wang Y, Lin Y, Li X, Wang Y, Bian J, Sun D, Kong L, Birnbaumer L, and Yang Y (2018). Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci 115(13): E2960–E2969. 10.1073/pnas.1720696115
- Aguilera M, Cerdà-Cuéllar M, and Martínez V (2015). Antibiotic-induced dysbiosis alters host-bacterial interactions and leads to colonic sensory and motor changes in mice. Gut Microbes 6(1): 10–23. 10.4161/19490976.2014.990790
- Allin KH, Nielsen T, and Pedersen O (2015). Mechanisms in endocrinology: Gut microbiota in patients with type 2 diabetes mellitus. Eur J Endocrinol 172(4): R167–R177. 10.1530/EJE-14-0874
- Schulz MD, Atay Ç, Heringer J, Romrig FK, Schwitalla S, Aydin B, Ziegler PK, Varga J, Reindl W, Pommerenke C, Salinas-Riester G, Böck A, Alpert C, Blaut M, Polson SC, Brandl L, Kirchner T, Greten FR, Polson SW, and Arkan MC (2014). High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 514(7253): 508–512. 10.1038/nature13398
- Serino M, Blasco-Baque V, Nicolas S, and Burcelin R (2014). Far from the Eyes, Close to the Heart: Dysbiosis of Gut Microbiota and Cardiovascular Consequences. Curr Cardiol Rep 16(11): 1–7. 10.1007/s11886-014-0540-1
- Vázquez-Castellanos JF, Serrano-Villar S, Latorre A, Artacho A, Ferrús ML, Madrid N, Vallejo A, Sainz T, Martínez-Botas J, Ferrando-Martínez S, Vera M, Dronda F, Leal M, Del Romero J, Moreno S, Estrada V, Gosalbes MJ, and Moya A (2015). Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunol 8(4): 760–772. 10.1038/mi.2014.107
- Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, and Ostatnikova D (2015). Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav 138: 179–187. 10.1016/j.physbeh.2014.10.033
- Yang Y, and Jobin C (2014). Microbial imbalance and intestinal pathologies: Connections and contributions. DMM Dis Model Mech 7(10): 1131–1142. 10.1242/dmm.016428
- Li J, Butcher J, Mack D, and Stintzi A (2015). Functional impacts of the intestinal microbiome in the pathogenesis of inflammatory bowel disease. Inflamm Bowel Dis 21(1): 139–153. 10.1097/MIB.0000000000000215
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, and Kaplan GG (2017). Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390(10114): 2769–2778. 10.1016/S0140-6736(17)32448-0
- Simian D, Fluxá D, Flores L, Lubascher J, Ibáñez P, Figueroa C, Kronberg U, Acuña R, Moreno M, and Quera R (2016). Inflammatory bowel disease: A descriptive study of 716 local Chilean patients. World J Gastroenterol 22(22): 5267–5275. 10.3748/wjg.v22.i22.5267
- Bellolio Roth F, Gómez J, and Cerda J (2017). Increase in Hospital Discharges for Inflammatory Bowel Diseases in Chile Between 2001 and 2012. Dig Dis Sci 62(9): 2311–2317. 10.1007/s10620-017-4660-5
- Cummings JRF, Cooney RM, Clarke G, Beckly J, Geremia A, Pathan S, Hancock L, Guo C, Cardon LR, and Jewell DP (2010). The genetics of NOD-like receptors in Crohn’s disease. Tissue Antigens 76(1): 48–56. 10.1111/j.1399-0039.2010.01470.x
- Lavoie S, Conway KL, Lassen KG, Jijon HB, Pan H, Chun E, Michaud M, Lang JK, Gallini Comeau CA, Dreyfuss JM, Glickman JN, Vlamakis H, Ananthakrishnan A, Kostic A, Garrett WS, and Xavier RJ (2019). The Crohn’s disease polymorphism, ATG16L1 T300A, alters the gut microbiota and enhances the local Th1/Th17 response. Elife 8(Cd): 1–28. 10.7554/eLife.39982
- Gholami M, M. Amoli M, and Sharifi F (2019). Overall corrections and assessments of “Correlations between TLR polymorphisms and inflammatory bowel disease: a meta-analysis of 49 case-control studies.” Immunol Res 67(4–5): 301–303. 10.1007/s12026-019-09092-w
- Sartor RB (2006). Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 3(7): 390–407. 10.1038/ncpgasthep0528
- Sepúlveda SE, Beltrán CJ, Peralta A, Rivas P, Rojas N, Figueroa C, Quera R, and Hermoso MA (2008). [Inflammatory bowel diseases: an immunological approach]. Rev Med Chil 136(3): 367–75. /S0034-98872008000300014
- Karczewski J, Poniedzialek B, Rzymski P, Rychlewska-Hańczewska A, Adamski Z, and Wiktorowicz K (2014). The effect of cigarette smoking on the clinical course of inflammatory bowel disease. Prz Gastroenterol 9(3): 153–159. 10.5114/pg.2014.43577
- Martin TD, Chan SSM, and Hart AR (2015). Environmental Factors in the Relapse and Recurrence of Inflammatory Bowel Disease: A Review of the Literature. Dig Dis Sci 60(5): 1396–1405. 10.1007/s10620-014-3437-3
- O’Toole A, and Korzenik J (2014). Environmental triggers for IBD. Curr Gastroenterol Rep 16(7). 10.1007/s11894-014-0396-y
- Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, and Doraé J (2009). Low counts of faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis 15(8): 1183–1189. 10.1002/ibd.20903
- Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, Ferrante M, Verhaegen J, Rutgeerts P, and Vermeire S (2014). A decrease of the butyrate-producing species roseburia hominis and faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63(8): 1275–1283. 10.1136/gutjnl-2013-304833
- Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, Bringer MA, Swidsinski A, Beaugerie L, and Colombel JF (2004). High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 127(2): 412–421. 10.1053/j.gastro.2004.04.061
- Small CLN, Reid-Yu SA, McPhee JB, and Coombes BK (2013). Persistent infection with Crohn’s disease-associated adherent-invasive Escherichia coli leads to chronic inflammation and intestinal fibrosis. Nat Commun 4: 1–12. 10.1038/ncomms2957
- Rigottier-Gois L (2013). Dysbiosis in inflammatory bowel diseases: The oxygen hypothesis. ISME J 7(7): 1256–1261. 10.1038/ismej.2013.80
- Albenberg L, Esipova T, Judge C, Bittinger K, Chen J, Laughlin A, Grunberg S, Baldassano R, Lewis J, Li H, Thom S, Bushman F, Vinogradov S, and Wu G (2015). Correlation Between Intraluminal Oxygen gradient and the microbiome. Gastroenterology 147(5): 1055–1063. 10.1053/j.gastro.2014.07.020.Correlation
- Lepage P, Hösler R, Spehlmann ME, Rehman A, Zvirbliene A, Begun A, Ott S, Kupcinskas L, Doré J, Raedler A, and Schreiber S (2011). Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology 141(1): 227–236. 10.1053/j.gastro.2011.04.011
- Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward D V., Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, and Huttenhower C (2012). Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 13(9). 10.1186/gb-2012-13-9-r79
- Zoetendal EG, Von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans ADL, and De Vos WM (2002). Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol 68(7): 3401–3407. 10.1128/AEM.68.7.3401-3407.2002
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, and Relman DA (2005). Diversity of the human intestinal microbial flora. Science 308(5728): 1635–8. 10.1126/science.1110591
- Ingala MR, Simmons NB, Wultsch C, Krampis K, Speer KA, and Perkins SL (2018). Comparing microbiome sampling methods in a wild mammal: Fecal and intestinal samples record different signals of host ecology, evolution. Front Microbiol 9: 1–13. 10.3389/fmicb.2018.00803
- Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z, Järnerot G, Tysk C, Jansson JK, and Engstrand L (2010). A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 139(6): 1844-1854.e1. 10.1053/j.gastro.2010.08.049
- Gevers D et al. (2014). The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 15(3): 382–392. 10.1016/j.chom.2014.02.005
- Vidal R, Ginard D, Khorrami S, Mora-Ruiz M, Munoz R, Hermoso M, Díaz S, Cifuentes A, Orfila A, and Rosselló-Móra R (2015). Crohn associated microbial communities associated to colonic mucosal biopsies in patients of the western Mediterranean. Syst Appl Microbiol 38(6): 442–452. 10.1016/j.syapm.2015.06.008
- Ludwig W et al. (2004). ARB: A software environment for sequence data. Nucleic Acids Res 32(4): 1363–1371. 10.1093/nar/gkh293
- Mora-Ruiz MDR, Font-Verdera F, Orfila A, Rita J, and Rosselĺo-Ḿora R (2016). Endophytic microbial diversity of the halophyte Arthrocnemum macrostachyum across plant compartments. FEMS Microbiol Ecol 92(9): 1–10. 10.1093/femsec/fiw145
- Wang F, Kaplan JL, Gold BD, Bhasin MK, Ward NL, Kellermayer R, Kirschner BS, Heyman MB, Dowd SE, Cox SB, Dogan H, Steven B, Ferry GD, Cohen SA, Baldassano RN, Moran CJ, Garnett EA, Drake L, Otu HH, Mirny LA, Libermann TA, Winter HS, and Korolev KS (2016). Detecting Microbial Dysbiosis Associated with Pediatric Crohn Disease Despite the High Variability of the Gut Microbiota. Cell Rep 14(4): 945–955. 10.1016/j.celrep.2015.12.088
- Schirmer M, Denson L, Vlamakis H, Franzosa EA, Thomas S, Gotman NM, Rufo P, Baker SS, Sauer C, Markowitz J, Pfefferkorn M, Oliva-Hemker M, Rosh J, Otley A, Boyle B, Mack D, Baldassano R, Keljo D, LeLeiko N, Heyman M, Griffiths A, Patel AS, Noe J, Kugathasan S, Walters T, Huttenhower C, Hyams J, and Xavier RJ (2018). Compositional and Temporal Changes in the Gut Microbiome of Pediatric Ulcerative Colitis Patients Are Linked to Disease Course. Cell Host Microbe 24(4): 600-610.e4. 10.1016/j.chom.2018.09.009
- Clooney AG, Eckenberger J, Laserna-Mendieta E, Sexton KA, Bernstein MT, Vagianos K, Sargent M, Ryan FJ, Moran C, Sheehan D, Sleator RD, Targownik LE, Bernstein CN, Shanahan F, and Claesson MJ (2020). Ranking microbiome variance in inflammatory bowel disease: a large longitudinal intercontinental study. Gut 70(3):499-510. 10.1136/gutjnl-2020-321106
- Alam MT, Amos GCA, Murphy ARJ, Murch S, Wellington EMH, and Arasaradnam RP (2020). Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathog 12(1): 1–8. 10.1186/s13099-019-0341-6
- Kedia S, Ghosh TS, Jain S, Desigamani A, Kumar A, Gupta V, Bopanna S, Yadav DP, Goyal S, Makharia G, Travis SPL, Das B, and Ahuja V (2020). Gut microbiome diversity in acute severe colitis is distinct from mild to moderate ulcerative colitis. J Gastroenterol Hepatol 36(3):731-739. 1–9. 10.1111/jgh.15232
- Fang X, Vázquez-Baeza Y, Elijah E, Vargas F, Ackermann G, Humphrey G, Lau R, Weldon KC, Sanders JG, Panitchpakdi M, Carpenter C, Jarmusch AK, Neill J, Miralles A, Dulai P, Singh S, Tsai M, Swafford AD, Smarr L, Boyle DL, Palsson BO, Chang JT, Dorrestein PC, Sandborn WJ, Knight R, and Boland BS (2020). Gastrointestinal Surgery for Inflammatory Bowel Disease Persistently Lowers Microbiome and Metabolome Diversity. Inflamm Bowel Dis 27(5):603-616. 10.1093/ibd/izaa262
- Dey N, Soergel DAW, Repo S, and Brenner SE (2013). Association of gut microbiota with post-operative clinical course in Crohn’s disease. BMC Gastroenterol 13(1): 1. 10.1186/1471-230X-13-131
- Sievert C, Parmer C, Hocking T, Chamberlain S, Corvellec M, and Despouy P (2020). Plotly: Create Interactive Web Graphics via “plotly.js.” Available at https://cran.r-project.org/package=plotly.
- R Core Team (2014). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at https://www.R-project.org/.
- Lloyd-Price J et al. (2019). Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569(7758): 655–662. 10.1038/s41586-019-1237-9
- Furusawa Y et al. (2013). Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504(7480): 446–450. 10.1038/nature12721
- Peng L, Li Z-R, Green RS, Holzman IR, and Lin J (2009). Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J Nutr 139(9): 1619–1625. 10.3945/jn.109.104638
- Friedman J, and Alm EJ (2012). Inferring Correlation Networks from Genomic Survey Data. PLoS Comput Biol 8(9): 1–11. 10.1371/journal.pcbi.1002687
- Kurtz ZD, Müller CL, Miraldi ER, Littman DR, Blaser MJ, and Bonneau RA (2015). Sparse and Compositionally Robust Inference of Microbial Ecological Networks. PLoS Comput Biol 11(5): 1–25. 10.1371/journal.pcbi.1004226
- Hughes ER, Winter MG, Duerkop BA, Spiga L, Furtado de Carvalho T, Zhu W, Gillis CC, Büttner L, Smoot MP, Behrendt CL, Cherry S, Santos RL, Hooper L V., and Winter SE (2017). Microbial Respiration and Formate Oxidation as Metabolic Signatures of Inflammation-Associated Dysbiosis. Cell Host Microbe 21(2): 208–219. 10.1016/j.chom.2017.01.005
- Henson MA, and Phalak P (2017). Microbiota dysbiosis in inflammatory bowel diseases: In silico investigation of the oxygen hypothesis. BMC Syst Biol 11(1): 1–15. 10.1186/s12918-017-0522-1
- Pascal V, Pozuelo M, Borruel N, Casellas F, Campos D, Santiago A, Martinez X, Varela E, Sarrabayrouse G, Machiels K, Vermeire S, Sokol H, Guarner F, and Manichanh C (2017). A microbial signature for Crohn’s disease. Gut 66(5): 813–822. 10.1136/gutjnl-2016-313235
- Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, Kotler E, Zur M, Regev-Lehavi D, Brik RBZ, Federici S, Cohen Y, Linevsky R, Rothschild D, Moor AE, Ben-Moshe S, Harmelin A, Itzkovitz S, Maharshak N, Shibolet O, Shapiro H, Pevsner-Fischer M, Sharon I, Halpern Z, Segal E, and Elinav E (2018). Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 174(6): 1388-1405.e21. 10.1016/j.cell.2018.08.041
- Becker C, Neurath MF, and Wirtz S (2015). The intestinal microbiota in inflammatory bowel disease. ILAR J 56(2): 192–204. 10.1093/ilar/ilv030
- Olaisen M, Flatberg A, Granlund A van B, Røyset ES, Martinsen TC, Sandvik AK, and Fossmark R (2020). Bacterial Mucosa-associated Microbiome in Inflamed and Proximal Noninflamed Ileum of Patients With Crohn’s Disease. Inflamm Bowel Dis 27(1):12-24. 10.1093/ibd/izaa107
- Högenauer C, Langner C, Beubler E, Lippe IT, Schicho R, Gorkiewicz G, Krause R, Gerstgrasser N, Krejs GJ, and Hinterleitner TA (2006). Klebsiella oxytoca as a Causative Organism of Antibiotic-Associated Hemorrhagic Colitis. N Engl J Med 355(23): 2418–2426. 10.1056/NEJMoa054765
- Chen J, Cachay ER, and Hunt GC (2004). Klebsiella oxytoca: a rare cause of severe infectious colitis: first North American case report. Gastrointest Endosc 60(1): 142–145. 10.1016/S0016-5107(04)01537-8
- Henke MT, Kenny DJ, Cassilly CD, Vlamakis H, Xavier RJ, and Clardy J (2019). Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc Natl Acad Sci U S A 116(26): 12672–12677. 10.1073/pnas.1904099116
- Hall AB, Yassour M, Sauk J, Garner A, Jiang X, Arthur T, Lagoudas GK, Vatanen T, Fornelos N, Wilson R, Bertha M, Cohen M, Garber J, Khalili H, Gevers D, Ananthakrishnan AN, Kugathasan S, Lander ES, Blainey P, Vlamakis H, Xavier RJ, and Huttenhower C (2017). A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med 9(1): 1–12. 10.1186/s13073-017-0490-5
- Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, and Florin THJ (2010). Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol 105(11): 2420–2428. 10.1038/ajg.2010.281
- Balish E, and Warner T (2002). Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol 160(6): 2253–2257. 10.1016/S0002-9440(10)61172-8
- Steck N, Hoffmann M, Sava IG, Kim SC, Hahne H, Tonkonogy SL, Mair K, Krueger D, Pruteanu M, Shanahan F, Vogelmann R, Schemann M, Kuster B, Sartor RB, and Haller D (2011). Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology 141(3): 959–971. 10.1053/j.gastro.2011.05.035
- Steck N, Mueller K, Schemann M, and Haller D (2012). Bacterial proteases in IBD and IBS. Gut 61(11): 1610–1618. 10.1136/gutjnl-2011-300775
- Wagner J, Short K, Catto-Smith AG, Cameron DJS, Bishop RF, and Kirkwood CD (2008). Identification and Characterisation of Pseudomonas 16S Ribosomal DNA from Ileal Biopsies of Children with Crohn’s Disease. PLoS One 3(10): 3(10):e3578. 10.1371/journal.pone.0003578
- Deng Q, and Barbieri JT (2008). Molecular mechanisms of the cytotoxicity of ADP-ribosylating toxins. Annu Rev Microbiol 62: 271–288. 10.1146/annurev.micro.62.081307.162848
- Nagao-Kitamoto H, and Kamada N (2017). Host-microbial cross-talk in inflammatory bowel disease. Immune Netw 17(1): 1–12. 10.4110/in.2017.17.1.1
- Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, and Nakaya R (1990). A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98(3): 694–702. 10.1016/0016-5085(90)90290-H
- Kolho KL, Korpela K, Jaakkola T, Pichai MVA, Zoetendal EG, Salonen A, and De Vos WM (2015). Fecal microbiota in pediatric inflammatory bowel disease and its relation to inflammation. Am J Gastroenterol 110(6): 921–930. 10.1038/ajg.2015.149
- Louis P, and Flint HJ (2009). Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 294(1): 1–8. 10.1111/j.1574-6968.2009.01514.x
- Takahashi K, Nishida A, Fujimoto T, Fujii M, Shioya M, Imaeda H, Inatomi O, Bamba S, Andoh A, and Sugimoto M (2016). Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 93(1): 59–65. 10.1159/000441768
- Segain JP, Raingeard de la Blétière D, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottière HM, and Galmiche JP (2000). Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut 47(3): 397–403. 10.1136/gut.47.3.397
- Cao Y, Shen J, and Ran ZH (2014). Association between faecalibacterium prausnitzii reduction and inflammatory bowel disease: A meta-analysis and systematic review of the literature. Gastroenterol Res Pract 2014:872725. 10.1155/2014/872725
- Galazzo G, Tedjo DI, Wintjens DSJ, Savelkoul PHM, Masclee AAM, Bodelier AGL, Pierik MJ, Jonkers DMAE, and Penders J (2019). Faecal Microbiota Dynamics and their Relation to Disease Course in Crohn’s Disease. J Crohn’s Colitis 13(10): 1273–1282. 10.1093/ecco-jcc/jjz049
- Ananthakrishnan AN (2020). Microbiome-Based Biomarkers for IBD. Inflamm Bowel Dis 26(10): 1463–1469. 10.1093/ibd/izaa071
- Guo S, Lu Y, Xu B, Wang W, Xu J, and Zhang G (2019). A simple fecal bacterial marker panel for the diagnosis of Crohn’s disease. Front Microbiol 10: 1–9. 10.3389/fmicb.2019.01306
- Wright EK, Kamm MA, Wagner J, Teo SM, Cruz P De, Hamilton AL, Ritchie KJ, Inouye M, and Kirkwood CD (2017). Microbial Factors Associated with Postoperative Crohn’s Disease Recurrence. J Crohns Colitis 11(2): 191–203. 10.1093/ecco-jcc/jjw136
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, and Weber CF (2009). Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75(23): 7537–7541. 10.1128/AEM.01541-09
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, Mcdonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, and Knight R (2010). correspondence QIIME allows analysis of high-throughput community sequencing data Intensity normalization improves color calling in SOLiD sequencing. Nat Publ Gr 7(5): 335–336. 10.1038/nmeth0510-335
- Pruesse E, Peplies J, and Glöckner FO (2012). SINA: Accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 28(14): 1823–1829. 10.1093/bioinformatics/bts252
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, and Glöckner FO (2013). The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res 41(D1): 590–596. 10.1093/nar/gks1219
- Dhariwal A, Chong J, Habib S, King IL, Agellon LB, and Xia J (2017). MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res 45(W1): W180–W188. 10.1093/nar/gkx295
- Warnes GR, Bolker B, Bonebakker L, Gentleman R, Huber W, Lumley T, Maechler M, Magnusson A, Moeller S, Schwartz M, and Venables B (2016). R Package ‘ gplots .’ Available at https://cran.r-project.org/web/packages/gplots/gplots.pdf.
- Menon R, Ramanan V, and Korolev KS (2018). Interactions between species introduce spurious associations in microbiome studies. PLOS Comput Biol 14(1): e1005939. 10.1371/journal.pcbi.1005939
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, and Huttenhower C (2011). Metagenomic biomarker discovery and explanation. Genome Biol 12(6):R60. 10.1186/gb-2011-12-6-r60
- Goksuluk D, Korkmaz S, Zararsiz G, and Karaagaoglu AE (2016). EasyROC: An interactive web-tool for roc curve analysis using r language environment. R J 8(2): 213–230. 10.32614/rj-2016-042
- Demsar J, and Zupan B (2013). Orange: data mining fruitful and fun. Informatica 37(1): 55.
–
SUPPLEMENTAL INFORMATION
![]() Download Supplemental Information
Download Supplemental Information
ACKNOWLEDGMENTS
We thank the National Laboratory for High Performance Computing, NLHPC (ECM-02), for sharing their server facilities, and Mauricio Cerda from the Center of Medical Informatics and Telemedicine (CIMT) at the University of Chile for his technical assis-tance. We thank Dr. Helen Lowry for her careful review of the manuscript and helpful discussions.
This study was supported by Fondo Nacional De Desarrollo Científico y Tecnológico FONDECYT grant 1161161 to R. Vidal, CONICYT-PCHA/2014-21140975 fellowship to N. Chamorro, FONDECYT 1120577 and 1170648 to Hermoso MA and the Spanish Ministry of Economy projects CLG2015 66686-C3-1-P to Rosselló-Mora R., as well as funds from the European Regional Development Fund (FEDER) and NSF Dimensions in Biodiversity grant OCE-1342694. Sup-port was also provided by a Millennium Science Initiative grant from the Ministry of Economy, Development and Tourism to Paredes-Sabja D.
COPYRIGHT
© 2021

Landscapes and bacterial signatures of mucosa-associated intestinal microbiota in Chilean and Spanish patients with inflammatory bowel disease by Chamorro et al. is licensed under a Creative Commons Attribution 4.0 International License.