Research Articles:
Microbial Cell, Vol. 11, No. 1, pp. 69 - 78; doi: 10.15698/mic2024.02.816
Replicative aging in yeast involves dynamic intron retention patterns associated with mRNA processing/export and protein ubiquitination
1 Tecnologico de Monterrey, Escuela de Ingeniería y Ciencias, Ave. Eugenio Garza Sada 2501, Monterrey, N.L., México.
Keywords: Saccharomyces cerevisiae, intron retention, replicative aging, mRNA processing, mRNA export, ubiquitination, transcription regulation.
Received originally: 30/10/2023 Received in revised form: 25/01/2024
Accepted: 01/02/2024
Published: 23/02/2024
Correspondence:
Christian Quintus Scheckhuber, Tel.: +52 81 2416 4423; c.scheckhuber@tec.mx
Conflict of interest statement: The authors declare no conflict of interest.
Please cite this article as: Jesús Gómez-Montalvo, Alvaro de Obeso Fernández del Valle, Luis Fernando De la Cruz Gutiérrez, José Mario González-Meljem and Christian Quintus Scheckhuber (2024). Replicative aging in yeast involves dynamic intron retention patterns associ-ated with mRNA processing/export and protein ubiquitination. Microbial Cell 11: 69-78. doi: 10.15698/mic2024.02.816
Abstract
Saccharomyces cerevisiae (baker´s yeast) has yielded relevant insights into some of the basic mechanisms of organismal aging. Among these are genomic instability, oxidative stress, caloric restriction and mitochondrial dysfunction. Several genes are known to have an impact on the aging process, with corresponding mutants exhibiting short- or long-lived phenotypes. Research dedicated to unraveling the underlying cellular mechanisms can support the identification of conserved mechanisms of aging in other species. One of the hitherto less studied fields in yeast aging is how the organism regulates its gene expression at the transcriptional level. To our knowledge, we present the first investigation into alternative splicing, particularly intron retention, during replicative aging of S. cerevisiae. This was achieved by utilizing the IRFinder algorithm on a previously published RNA-seq data set by Janssens et al. (2015). In the present work, 44 differentially retained introns in 43 genes were identified during replicative aging. We found that genes with altered intron retention do not display significant changes in overall transcript levels. It was possible to functionally assign distinct groups of these genes to the cellular processes of mRNA processing and export (e.g., YRA1) in early and middle-aged yeast, and protein ubiquitination (e.g., UBC5) in older cells. In summary, our work uncovers a previously unexplored layer of the transcriptional program of yeast aging and, more generally, expands the knowledge on the occurrence of alternative splicing in baker´s yeast.
INTRODUCTION
Organismal aging is a universal phenomenon characterized by a progressive and irreversible decline in physiological functions, accompanied by increased morbidity and mortality [1]. Extensive research across various model organisms and systems has aimed to decipher the underlying processes of aging [2]. This intricate phenomenon is governed by a complex network of molecular pathways that can exhibit both species-specific traits and conserved mechanisms [3]. Understanding the fundamental mechanisms that drive aging holds paramount importance, offering insights into the aging process across different systems [4].
–
The unicellular ascomycete Saccharomyces cerevisiae has emerged as a valuable model organism for investigating the mechanistic basis of aging [5]. Replicative aging in S. cerevisiae is defined by the limited number of daughter cells a mother cell can produce through the budding process [6]. It is accompanied by an increase in the time required for mother cells to produce subsequent daughter cells, ultimately leading to the cessation of daughter cell production and the demise of the senescent mother cell [7]. Identification of genes regulating or at least influencing the aging process of S. cerevisiae is facilitated by the fact that it is characterized by a streamlined genome of approximately 12.07 Mb distributed across 16 linear chromosomes [8][9]. Within this genome, a set of over 6000 genes has been identified, of which only around 5% harbor introns, amounting to 296 introns from 287 genes—a notably sparse distribution of introns throughout the yeast’s genetic landscape [10], while a prior report suggested that S. cerevisiae contained only 253 introns with a mere six genes featuring two introns [11].
–
The influence of mRNA levels on protein expression is well-recognized, encompassing not only gene expression but also RNA decay rates [12]. RNA decay, often impacted by premature translation termination [13], becomes a pivotal aspect in the fate of messenger RNA molecules. Of particular note is alternative splicing (AS)—an intricate process yielding either novel proteins or mRNA transcripts prone to degradation via the nonsense-mediated mRNA decay (NMD) pathway. In the context of yeast, the influence of AS has been discerned since 1990, with different mRNA sequences orchestrating this process [14].
–
The SUS1 gene, remarkably bearing two introns, offers an intriguing link between AS and its functional outcomes. This gene, pivotal in mRNA transport and histone H2B deubiquitination, provides an exemplar of how AS can impact gene expression and subsequent cellular processes [15]. Although the prevalence of AS in fungi is generally overshadowed by metazoan proteome diversification mechanisms, sporadic instances such as the sub-functionalization of the SKI7/HBS1 gene demonstrate its relevance [16].
–
Recent research has unveiled a nuanced perspective on AS in S. cerevisiae. While frequent, AS in yeast is often modulated by RNA degradation, predominantly serving to regulate transcript abundance rather than expanding the proteome [17]. The SUS1 gene again emerges as a case in point, where the non-canonical sequences within its first intron led to retention and subsequent degradation—a regulatory strategy that underscores the intricacies of splicing [15].
–
Intriguingly, the prevalence of introns in Ascomycetes, including S. cerevisiae, has been implicated in homologous recombination with reverse-transcribed RNA containing introns, reflecting the dynamic interplay between introns and genome stability [18]. Ubiquitin-like proteins, exemplified by Hub1p, contribute to AS modulation, interacting with spliceosomes and influencing their activity [19].
–
While instances of protein diversity resulting from AS have been documented in Ascomycetes, the intricate regulatory mechanisms underlying these events are still being unraveled. YRA1, a gene involved in mRNA export, utilizes intron retention (IR) as a mechanism for autoregulation, shedding light on the multifaceted roles of AS in cellular control [20]. Similarly, the PTC7 gene undergoes AS, generating distinct protein isoforms with disparate subcellular locations—a process with implications for cellular function and compartmentalization [21].
–
Beyond S. cerevisiae, other yeast species such as Yarrowia lipolytica and Schizosaccharomyces pombe have exhibited AS capabilities, further emphasizing the evolutionary significance of this process [22][23][24]. The prevalence of IR has been identified as a hallmark of genome-wide age-related experiments, extending to organisms like Caenorhabditis elegans, where IR pertains to metabolic processes including carbohydrate transport and lipid catabolism, even under dietary restrictions [25][26][27].
–
In this study, we delve into the intricate landscape of AS in the context of yeast aging, particularly focusing on the model organism S. cerevisiae. By exploring the complexities of IR and functional implications, we aim to increase our understanding of AS during the aging process of yeast.
RESULTS
To investigate the occurrence of IR during replicative yeast aging, we analyzed public poly-A RNA-seq data derived from yeast mother cells isolated at different replicative ages: 0 h, 7.8 h, 17.8 h, 45.4 h and 72.3 h (collectively referred to as Janssens data set [28] hereinafter). To generate this data set, Janssens et al. used a column system in which yeast mother cells labeled with iron beads were retained using a magnet, while the generated daughter cells were continuously washed away. Specifically, we used the data labeled as Mix 2 (ArrayExpress accession: E-MTAB-3605) as it corresponds to the column fraction enriched in aged yeast mother cells. As a quality control measure, we confirmed that the gene expression values obtained through our edgeR analysis closely matched those initially reported by Janssens et al. [28] (Fig. S1A). We further verified that the Janssens data set evinced transcriptional changes associated with replicative aging. Gene set enrichment analysis (GSEA) indicated enrichment of a transcriptional signature that has been identified in wild type and mutant yeast models of replicative aging [29] (Fig. S1B). Thus, we confirmed that we analyzed representative data of the transcriptional program of replicative yeast aging.
–
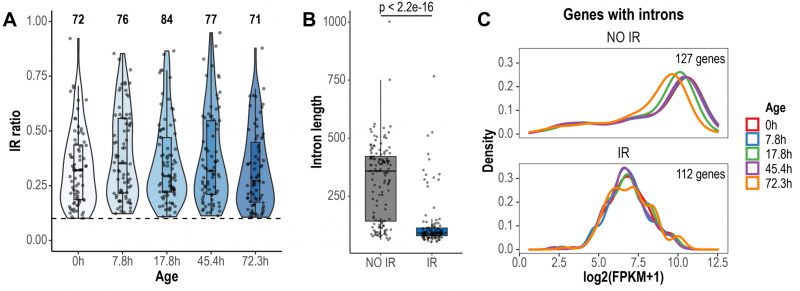
We conducted a global analysis of retained introns using IRFinder [30]. IRFinder uses the IR ratio metric, which represents the proportion of transcripts retaining introns for a given gene. As reported in other publications [30][31], we considered an intron as retained if it was estimated to be present in at least 10% of the transcripts (IR ratio > 0.1). In total, 116 retained introns in 112 genes were identified across all aging time points (Fig. 1A). Most retained introns displayed IR ratio values between 0.1-0.5 (Fig. 1A). Previous reports have highlighted that retained introns are shorter in length and have higher GC content, compared to non-retained introns [31][32]. We found that introns identified as retained in the Janssens data set were significantly shorter than non-retained introns (retained introns average length = 127 bp, non-retained introns average length = 314 bp, p < 2.2e-16, Wilcoxon rank sum test; Fig. 1B). However, GC content was not different between retained and non-retained introns (retained introns average GC content = 32.5%, non-retained introns average GC content = 33%, p = 0.278, Wilcoxon rank sum test). Genes expressing IR transcripts displayed lower transcript levels than genes that did not retain introns (Fig. 1C). Of note, this previously unexplored feature of IR in yeast goes in line with previous observations in human cells [33].
–
–
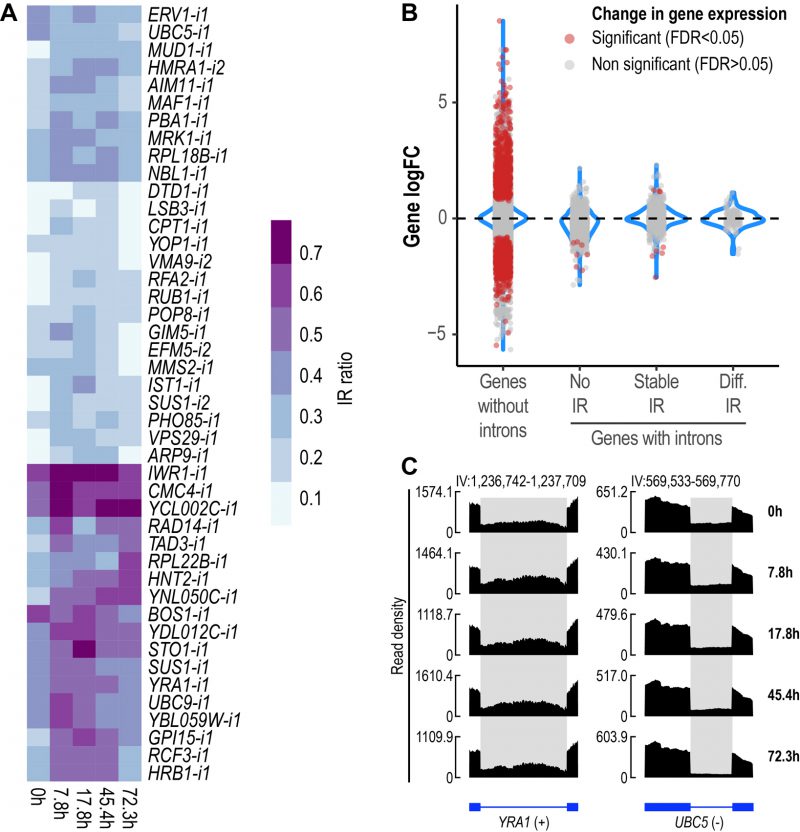
We then sought to identify introns with altered retention levels during replicative aging. IR was found to be a dynamic event during replicative aging. Overall, 44 differentially retained introns in 43 genes were identified across all aging time points (Fig. 2A, Supplemental Table 1). To gain further insight into the relevance of IR as part of the transcriptional program of replicative aging in yeast, we assessed the occurrence of other AS events using rMATS [34]. We analyzed exon skipping and alternative 5′ and 3′ splice sites, all of which are known to occur in S. cerevisiae [35][36]. Apart from IR, we only found alternative 3′ splice sites to be altered (A3SS), albeit at a lower proportion (A3SS = 14 events in ten genes) (Fig. S2). These results indicate that IR represents the predominant type of AS during replicative aging in yeast.
–
–
Changes in IR levels can affect gene expression [37]. To further explore whether genes with differentially retained introns displayed altered transcript levels, we divided yeast genes into four categories (Fig. 2B): genes without introns (or single-exon genes), genes with non-retained introns (No IR), genes showing stable IR levels (Stable IR), and genes with differentially retained introns (Diff. IR). Notably, it was only the latter group that did not display any significant changes in transcript levels during yeast aging (Fig. 2B). This observation indicates that during replicative aging some genes may be functionally altered via IR without displaying changes in gene expression.
–
We identified genes showing differential IR that have been previously implicated in replicative yeast aging, e.g., LSB3, PHO85 and HRB1, whose corresponding mutants display altered longevity [38]. However, we detected various other genes that have not been formally associated with aging in yeast, two of which are exemplified in Fig. 2C. YRA1, which encodes an RNA-binding protein involved in the nuclear export of mRNAs, displayed increased IR levels during the early and middle stages of aging (Fig. 2C, left). Conversely, UBC5, which encodes a ubiquitin-conjugating enzyme, showed decreased IR as age progressed (Fig. 2C, right). The RNA-seq tracks of all the other differentially retained introns are shown in Fig S3.
–
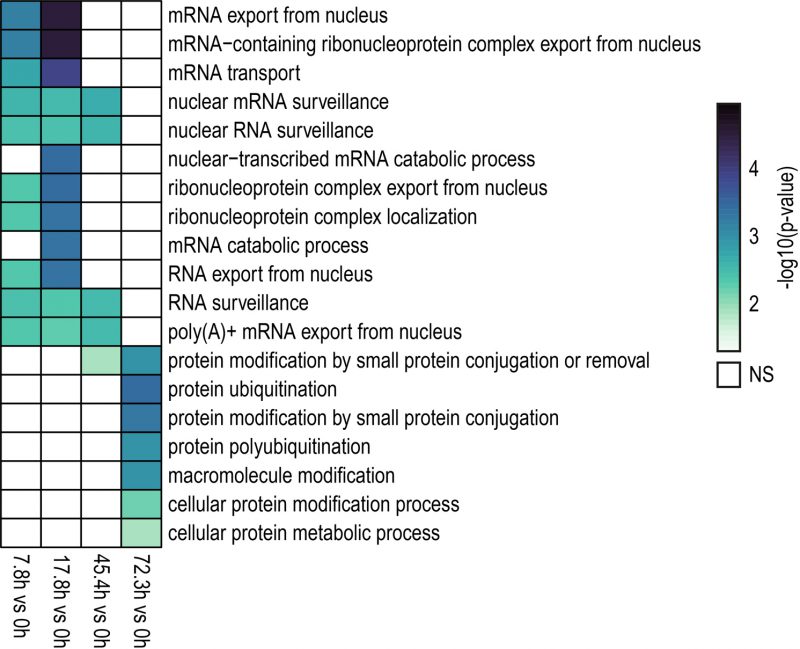
A gene ontology (GO) analysis was conducted to identify enriched biological processes among genes showing differential IR levels during yeast aging (Fig. 3). The GO analysis revealed that during the early stages of yeast aging, genes showing differential IR were primarily associated with mRNA processing and export. On the other hand, increased yeast age was associated with altered IR levels in genes related to protein ubiquitination. Altogether, the GO analysis suggests that these processes may be functionally altered by IR during yeast aging. In fact, this may go in line with previous observations in which protein overabundance and uncoupled transcript and protein levels were observed during replicative yeast aging [28].
–
–
Our results highlight the occurrence of dynamically altered IR events during replicative aging in yeast. To further expand our findings, we analyzed two additional poly-A RNA-seq data sets of aged S. cerevisiae mother cells reported by Paukštytė et al. [39] (replicative age: 6 h) and Hendrickson et al. [29] (replicative ages: 20 h, 40 h). To generate these data sets, the authors labeled yeast mother cells with iron beads and recovered them using a magnet at different replicative ages, in a similar way to the approach used by Janssens et al. [28]. We identified 21 and 17 differentially retained introns in the Paukštytė (Fig. S4A) and Hendrickson (Fig. S4B) data sets, respectively, various of which were shared with the Janssens data set (Fig. S4C). Most differential IR events (75%) shared with the Janssens data set displayed the same pattern of increased retention during aging. Interestingly, we found overlap of differential IR events in genes associated with mRNA processing and export (e.g., YRA1 and STO1) and protein ubiquitination and the proteasome (e.g., UBC9 and PBA1). These results reinforce the fact that IR is an altered component of the transcriptional program of replicative yeast aging. The data of all the differentially retained introns identified in this study can be found in Supplemental Table 1.
DISCUSSION
In this study, our goal was to analyze yeast transcriptome data for the occurrence of IR during replicative aging. Towards this end, we primarily utilized the high-quality RNA-seq data set previously reported by Janssens et al. [28]. For generating this data set, the authors effectively isolated aged yeast mother cells using a then novel column system and profiled their transcriptome at different time points. With this data set, Janssens et al. originally reported a valuable analysis of gene expression changes during replicative yeast aging. We expanded on the data previously presented by Janssens et al. by exploring whether old cells are marked by a different occurrence of IR compared to young cells.
–
IR has been relatively poorly studied in the context of aging. Only a few studies highlight the importance of IR as part of the transcriptional landscape of aging and age-related diseases [32][40][41][42][43]. For instance, analysis of Drosophila heads, and human and mouse brains revealed increased IR in aged organisms [32]. Increased IR is also observed in the frontal cortex and cerebellum of Alzheimer’s disease patients [32], as well as in a large number of cancer types [44]. More recently, IR has been proposed as a pre-symptomatic marker of aging [40].
–
In the present study, we found that IR is dynamically altered in yeast during replicative aging (Fig. 2A). Notably, genes with differentially retained introns did not display altered transcript levels (Fig. 2B). In various studies, it has been frequently observed that changes in IR levels are inversely related to changes in gene expression, e.g., increased IR results in decreased gene expression and vice versa [42][45][46][47]. However, this was not the case in aged Drosophila heads, and aged human and mouse brains, in which genes with altered IR did not display changes in gene expression [32], which is in line with our findings for yeast aging. Based on these observations, we underscore the fact that genes with altered IR, but unaltered transcript levels, may be overlooked when conducting conventional transcriptomic analyses in aging studies. Consequently, genes potentially contributing to the onset and development of aging via IR could be potentially ignored.
–
An important aspect of replicative aging in yeast is the increased time mother cells take to generate daughter cells. This is ultimately accompanied by a decline in the replicative capacity of aged cells [48]. In other organisms it has been observed that, while cells that can transition into a proliferative state display lower IR levels, cells transitioning towards post-mitotic states show increased IR [49]. In the present study, of the 44 differentially retained introns that we identified in the Janssens data set, 32 displayed increased IR levels during the time course of aging (Fig. 2A, Supplemental Table 1). Increased IR during replicative yeast aging may be a consequence of the ongoing transition towards the cell state of reduced replicative capacity. Nonetheless, further research is required to unveil the underlying mechanisms driving increased IR during yeast aging.
–
We found that genes showing differential IR during replicative aging in yeast were primarily associated with the processes of mRNA processing and export, and protein ubiquitination (Fig. 3). Interestingly, genes with altered IR in the aged mouse brain and in the liver of a mouse model of premature aging were involved in similar processes [32][40][41]. These observations underscore the involvement of IR in these cellular functions as part of the transcriptional program of aging across species.
–
Various mRNA export genes that we identified, such as SUS1, STO1, YRA1 and HRB1 displayed increased IR during yeast aging (Fig. 2A). The Yra1p and Hrb1p proteins work conjointly during transcription and mRNA export [50]. IR in YRA1 transcripts introduces a premature termination codon that triggers their degradation as a mechanism to regulate Yra1p protein levels [20]. Yra1p is a key mRNA export factor in yeast as YRA1 mutants display marked export defects [51][52]. In contrast, Δhrb1 mutants do not show impaired mRNA export, but rather aberrant leakage of IR transcripts into the cytoplasm [53]. This underscores the role of Hrb1p in nuclear RNA surveillance and mRNA export quality control. Notably, HRB1 has been previously associated with aging in S. cerevisiae as Δhrb1 mutants display increased longevity [38]. Several studies in other species, such as human and mouse, have highlighted the occurrence of IR in RNA-binding protein genes involved in various functions, such as splicing, mRNA export, and polyadenylation of mRNA [44][54][55][56][57][58]. For instance, in human motor neurons carrying mutations that cause amyotrophic lateral sclerosis, a disease largely dependent on age, the most prominent IR event was observed in the SFPQ gene, which is involved in transcription and splicing [57].
–
On the other hand, we found that protein ubiquitination genes, such as UBC5 and MMS2 displayed decreased IR during the latest aging time point analyzed here (Fig. 2A). In contrast, IR levels in UBC9 transcripts peaked during early stages of aging and returned to “basal” levels at later time points. In addition, PBA1 transcripts, which encode a chaperone involved in the assembly of the 20S proteasome, also displayed increased IR during aging. The ubiquitin-proteasome pathway (UPP) is known to be impaired during aging [59]. In fact, loss of proteostasis is a recognized hallmark of aging and age-related diseases, such as Alzheimer’s and Parkinson’s disease [60]. In yeast, impaired proteasome activity and increased accumulation of ubiquitinated proteins are observed during aging [61]. Moreover, yeast with enhanced proteasome function display increased longevity [62]. Although UBC5 has not been formally associated with yeast aging, it has been proposed as a potential pro-longevity gene using predictive models based on functional annotation and gene expression data [63].
–
Previously, Janssens et al. [28] reported an intriguing finding during replicative yeast aging in that they observed that as age increases there is less agreement between transcript and protein levels, meaning that transcriptional changes did not correlate with alterations observed in the proteome. We suggest that altered mRNA export and protein degradation as a result of the dynamic IR patterns identified here may contribute to the discrepancies between transcript and protein levels previously observed in replicative aging in yeast [28]. Although it has not yet been studied whether altered IR in mRNA export genes impairs the transport of transcripts into the cytoplasm, previous observations in S. cerevisiae have highlighted that deletion of the YRA1 intron results in nuclear accumulation of mRNAs and slowed growth [64]. Therefore, dysregulation of IR events in genes here identified such as YRA1, HRB1 or SUS1 could have profound consequences in the availability of cytoplasmic transcripts ready for translation, thus potentially affecting the proteome of aged yeast.
–
By comparing various data sets of replicative aged yeast, we found increased IR in GPI15 transcripts as a shared event in all data sets (Fig. S4C). GPI15 encodes a protein involved in the biosynthesis of glycosylphosphatidylinositol anchors, which are post-translational modifications added to some membrane proteins [65]. While there is no reported association between GPI15 and yeast aging, a functionally related gene GPI7 has been predicted to impact yeast lifespan [66]. Furthermore, in another study in which the unfolded protein response pathway was compromised in yeast, the expression of GPI15, and other functionally related genes, was found to be altered [67]. It was hypothesized that changes in the expression of these genes may aid in counteracting endoplasmic reticulum stress [67]. Notably, the unfolded protein response, and consequently proteostasis, are impaired during aging, resulting in increased endoplasmic reticulum stress [68][69], in which IR in GPI15 transcripts may play a role as yeast age progresses. Further research into the occurrence of IR in GPI15 transcripts during yeast aging is required to better understand the functional consequences of this widespread event.
–
One limitation of our study is the fact that we utilized the raw, “mixed” RNA-seq data reported by Janssens et al. [28]. Originally, the authors conducted an “unmixing” step, in which they removed the potential influence of the transcriptome of daughter or dead cells present in the samples that were enriched for aged yeast mother cells. However, during the “unmixing” step the intron information was lost as the samples were unmixed at the gene expression level. For our study, we required the information of introns which was still available in the raw data set. Nonetheless, we found that IR is altered in other studies of replicative yeast aging and some of these differential IR events were shared with the Janssens data set (Fig. S4), further supporting our observations that IR is part of the transcriptional program of replicative aging in yeast.
–
Our work highlights the role of changes in transcriptional regulation during aging. This might also hold true for other organisms besides yeast. As outlined above, it has been shown that IR is increased in Drosophila heads, and human and mouse brains during aging [32]. Since yeast is characterized by chronological aging and replicative aging, it would be straightforward to analyze IR during stationary phase survival, which only depends on the availability of a high-quality transcriptome data set. We assume that several important regulatory principles can be identified by our approach, which ultimately might even guide interventions into the aging process.
MATERIALS AND METHODS
Data collection and processing
RNA-seq data from replicative aged yeast was retrieved from the study by Janssens et al. [28] (strain: YSBN6; ArrayExpress accession: E-MTAB-3605). This dataset was selected since it met various criteria for the proper analysis of AS and IR in yeast aging: (1) enrichment and isolation of aged yeast mother cells was effectively conducted, (2) poly-A RNA was enriched during library preparation, and (3) sequencing was paired-end. Five aging time points with two replicates each were analyzed: 0 h, 7.8 h, 17.8 h, 45.4 h and 72.3 h. Two additional RNA-seq data sets of wild type replicative aged yeast that met the above-mentioned criteria were obtained from the studies by Paukštytė et al. [39] (strain: BY4741; replicative age: 6h; Harvard Dataverse: https://doi.org/10.7910/DVN/DUOBUD) and Hendrickson et al. [29] (strain: DBY12000; replicative ages: 20h and 40h; GEO accession: GSE118581). Read quality was assessed with FastQC v0.12.1. Low quality bases (quality score < 30) and sequencing adapters were trimmed using Cutadapt v4.4. STAR v2.7.10b [70] was used to map the filtered reads against the R64 S. cerevisiae genome. Since the size of the yeast genome is 12.1 Mb, the parameter –genomeSAindexNbases was set to 10 when indexing the genome.
–
Differential gene expression analysis
Gene counts were generated using featureCounts (Rsubread package v2.14.2) together with the S. cerevisiae ENSEMBL gene annotation (release 109). Normalization of gene counts, computation of FPKM values, and differential gene expression analysis were conducted with edgeR v3.42.4 [71]. Genes showing FDR < 0.05 were considered as differentially expressed.
–
Correlation analysis
To make sure that the gene expression data here generated using our pipeline was consistent with the original raw analysis conducted by Janssens et al. [28], a correlation analysis was conducted. Log2 fold change values for all contrasts against 0h were computed using the raw FPKM values reported in Janssens et al. [28] (source data 2); the same was performed with the FPKM values here generated. Spearman correlation analysis was conducted for genes identified as differentially expressed in our pipeline (7.8 h vs 0 h, 122 genes; 17.8 h vs 0 h, 186 genes; 45.4 h vs 0 h, 301 genes; 72.3 h vs 0 h, 546 genes).
–
Gene set enrichment analysis of aging genes
Custom yeast aging gene sets were generated using the top 100 up- and downregulated genes reported in the analysis by Hendrickson et al. [29], in which DBY12000 wild type (WT) and three mutant (Δsir2, Δubr2, Δfob1) yeast strains were profiled during replicative aging. edgeR normalized expression values for all genes here analyzed were introduced to the GSEA tool v4.2.2 [72]. GSEA was run by setting the Metric for ranking genes parameter to log2_Ratio_of_Classes as suggested when analyzing experiments with less than 3 replicates. Significant gene sets were considered as those with p-value < 0.05 and FDR < 0.25.
–
Intron retention analysis
IRFinder v1.3.1 [30] was used for the analysis of retained introns. IRFinder uses the IR ratio metric to estimate IR levels and is equivalent to the proportion of transcripts retaining introns. IRFinder reference was generated using the S. cerevisiae ENSEMBL gene annotation (release 109). IR was assessed in 253 introns of 243 genes. In the case of the Janssens data set (two replicates per time point), differential IR analysis was conducted using the analysisWithLowReplicates.pl script (Audic and Claverie test [73]). For the Paukštytė (four replicates per time point) and Hendrickson data sets (three replicates per time point), differential IR analysis was conducted using the Generalized Linear Model approach with DESeq2 [74] (Likelihood Ratio Test). Differentially retained introns were considered as those showing |ΔIR ratio| > 0.1 and p-value < 0.05.
–
Alternative splicing analysis
rMATS v4.1.2 [34] was used to identify AS events other than IR, e.g., exon skipping and alternative 5′ and 3′ splice sites. rMATS was run using the —novelSS parameter. The output was further filtered using maser v1.18.0 to keep AS events with at least five reads supporting their expression, and a modified version of the topEvents() function was used to identify differential AS events as those showing |ΔPSI| > 0.1 and p-value < 0.05 (PSI, Percent Spliced In).
–
Gene ontology analysis of genes with differential IR
Gene ontology (GO) analysis was conducted for genes showing differentially retained introns using DAVID (https://david.ncifcrf.gov/) [75]. Significantly enriched biological processes (p < 0.05, Fisher’s Exact test) were identified using the GOTERM_BP_ALL category.
REFERENCES
- Kirkwood TB, and Austad SN (2000). Why do we age? Nature 408(6809): 233–238. 10.1038/35041682
- Minois N (2006). How should we assess the impact of genetic changes on ageing in model species? Ageing Res Rev 5(1): 52–59. 10.1016/j.arr.2005.08.001
- Kirkwood TB (2002). Molecular gerontology. J Inherit Metab Dis 25(3): 189–196. 10.1023/a:1015625811569
- da Costa JP, Vitorino R, Silva GM, Vogel C, Duarte AC, and Rocha-Santos T (2016). A synopsis on aging-Theories, mechanisms and future prospects. Ageing Res Rev 29: 90–112. 10.1016/j.arr.2016.06.005
- Kaeberlein M (2010). Lessons on longevity from budding yeast. Nature 464(7288): 513–519. 10.1038/nature08981
- Jazwinski SM, Egilmez NK, and Chen JB (1989). Replication control and cellular life span. Exp Gerontol 24(5–6): 423–436. 10.1016/0531-5565(89)90049-1
- Mortimer RK, and Johnston JR (1959). Life span of individual yeast cells. Nature 183(4677): 1751–1752. 10.1038/1831751a0
- Dujon B (1996). The yeast genome project: what did we learn? Trends Genet 12(7): 263–270. 10.1016/0168-9525(96)10027-5
- Belda I, Ruiz J, Santos A, Van Wyk N, and Pretorius IS (2019). Saccharomyces cerevisiae. Trends Genet 35(12): 956–957. 10.1016/j.tig.2019.08.009
- Neuvéglise C, Marck C, and Gaillardin C (2011). The intronome of budding yeasts. C R Biol 334(8–9): 662–670. 10.1016/j.crvi.2011.05.015
- Barrass JD, and Beggs JD (2003). Splicing goes global. Trends Genet 19(6): 295–298. 10.1016/S0168-9525(03)00091-X
- Ross J (1989). The Turnover of Messenger RNA. Sci Am 260(4): 48–55. 10.1038/scientificamerican0489-48
- Peltz SW, He F, Welch E, and Jacobson A (1994). Nonsense-mediated mRNA Decay in Yeast. In: Cohn WE, Moldave K, editors. Prog. Nucleic Acid Res. Mol. Biol. Academic Press; pp 271–298
- Jacobson A, Brown AH, Donahue JL, Herrick D, Parker R, and Peltz SW (1990). Regulation of mRNA Stability in Yeast. In: McCarthy JEG, Tuite MF, editors. Post-Transcriptional Control of Gene Expr. Springer, Berlin, Heidelberg; pp 45–54
- Hossain MA, Rodriguez CM, and Johnson TL (2011). Key features of the two-intron Saccharomyces cerevisiae gene SUS1 contribute to its alternative splicing. Nucleic Acids Res 39(19): 8612–8627. 10.1093/nar/gkr497
- Marshall AN, Montealegre MC, Jiménez-López C, Lorenz MC, and van Hoof A (2013). Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal Proteomes. PLoS Genet 9(3): e1003376. 10.1371/journal.pgen
- Kawashima T, Douglass S, Gabunilas J, Pellegrini M, and Chanfreau GF (2014). Widespread Use of Non-productive Alternative Splice Sites in Saccharomyces cerevisiae. PLoS Genet 10(4): e1004249. 10.1371/journal.pgen.1004249
- Derr LK, and Strathern JN (1993). A role for reverse transcripts in gene conversion. Nature 361(6408): 170–173. 10.1038/361170a0
- Mishra SK, Ammon T, Popowicz GM, Krajewski M, Nagel RJ, Ares M, Holak TA, and Jentsch S (2011). Role of the ubiquitin-like protein Hub1 in splice-site usage and alternative splicing. Nature 474(7350): 173–178. 10.1038/nature10143
- Preker PJ, Kim KS, and Guthrie C (2002). Expression of the essential mRNA export factor Yra1p is autoregulated by a splicing-dependent mechanism. RNA 8(8): 969–980. 10.1017/s1355838202020046
- Juneau K, Nislow C, and Davis RW (2009). Alternative Splicing of PTC7 in Saccharomyces cerevisiae Determines Protein Localization. Genetics 183(1): 185–194. 10.1534/genetics.109.105155
- Kabran P, Rossignol T, Gaillardin C, Nicaud JM, and Neuvéglise C (2012). Alternative Splicing Regulates Targeting of Malate Dehydrogenase in Yarrowia lipolytica. DNA Res 19(3): 231–244. 10.1093/dnares/dss007
- Mekouar M, Blanc-Lenfle I, Ozanne C, Da Silva C, Cruaud C, Wincker P, Gaillardin C, and Neuvéglise C (2010). Detection and analysis of alternative splicing in Yarrowia lipolytica reveal structural constraints facilitating nonsense-mediated decay of intron-retaining transcripts. Genome Biol 11(6): R65. 10.1186/gb-2010-11-6-r65
- Montañés JC, Huertas M, Moro SG, Blevins WR, Carmona M, Ayté J, Hidalgo E, and Albà MM (2022). Native RNA sequencing in fission yeast reveals frequent alternative splicing isoforms. Genome Res 32(6): 1215–1227. 10.1101/gr.276516.121
- Heintz C, Doktor TK, Lanjuin A, Escoubas CC, Zhang Y, Weir HJ, Dutta S, Silva-García CG, Bruun GH, Morantte I, Hoxhaj G, Manning BD, Andresen BS, and Mair WB (2017). Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. elegans. Nature 541(7635): 102–106. 10.1038/nature20789
- Rollins JA, Shaffer D, Snow SS, Kapahi P, and Rogers AN (2019). Dietary restriction induces posttranscriptional regulation of longevity genes. Life Sci Alliance 2(4): e201800281. 10.26508/lsa.201800281
- Tabrez SS, Sharma RD, Jain V, Siddiqui AA, and Mukhopadhyay A (2017). Differential alternative splicing coupled to nonsense-mediated decay of mRNA ensures dietary restriction-induced longevity. Nat Commun 8(1): 306. 10.1038/s41467-017-00370-5
- Janssens GE, Meinema AC, González J, Wolters JC, Schmidt A, Guryev V, Bischoff R, Wit EC, Veenhoff LM, and Heinemann M (2015). Protein biogenesis machinery is a driver of replicative aging in yeast. Elife 4: e08527. 10.7554/eLife.08527
- Hendrickson DG, Soifer I, Wranik BJ, Kim G, Robles M, Gibney PA, and McIsaac RS (2018). A new experimental platform facilitates assessment of the transcriptional and chromatin landscapes of aging yeast. Elife 7: e39911. 10.7554/eLife.39911
- Middleton R, Gao D, Thomas A, Singh B, Au A, Wong JJ, Bomane A, Cosson B, Eyras E, Rasko JEJ, and Ritchie W (2017). IRFinder: Assessing the impact of intron retention on mammalian gene expression. Genome Biol 18(1): 51. 10.1186/s13059-017-1184-4
- Schmitz U, Pinello N, Jia F, Alasmari S, Ritchie W, Keightley MC, Shini S, Lieschke GJ, Wong JJ, and Rasko JEJ (2017). Intron retention enhances gene regulatory complexity in vertebrates. Genome Biol 18(1): 216. 10.1186/s13059-017-1339-3
- Adusumalli S, Ngian ZK, Lin WQ, Benoukraf T, and Ong CT (2019). Increased intron retention is a post-transcriptional signature associated with progressive aging and Alzheimer’s disease. Aging Cell 18(3): e12928. 10.1111/acel.12928
- Wong JJ, Gao D, Nguyen TV, Kwok CT, van Geldermalsen M, Middleton R, Pinello N, Thoeng A, Nagarajah R, Holst J, Ritchie W, and Rasko JEJ (2017). Intron retention is regulated by altered MeCP2-mediated splicing factor recruitment. Nat Commun 8: 15134. 10.1038/ncomms15134
- Shen S, Park JW, Lu ZX, Lin L, Henry MD, Wu YN, Zhou Q, and Xing Y (2014). rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc Natl Acad Sci USA 111(51): E5593–E5601. 10.1073/pnas.1419161111
- Schreiber K, Csaba G, Haslbeck M, and Zimmer R (2015). Alternative Splicing in Next Generation Sequencing Data of Saccharomyces cerevisiae. PLoS One 10(10): e0140487. 10.1371/journal.pone.0140487
- Egecioglu DE, Kawashima TR, and Chanfreau GF (2012). Quality control of MATa1 splicing and exon skipping by nuclear RNA degradation. Nucleic Acids Res 40(4): 1787–1796. 10.1093/nar/gkr864
- Grabski DF, Broseus L, Kumari B, Rekosh D, Hammarskjold ML, and Ritchie W (2021). Intron retention and its impact on gene expression and protein diversity: A review and a practical guide. Wiley Interdiscip Rev RNA 12(1): e1631. 10.1002/wrna.1631
- Smith ED, Tsuchiya M, Fox LA, Dang N, Hu D, Kerr EO, Johnston ED, Tchao BN, Pak DN, Welton KL, Promislow DE, Thomas JH, Kaeberlein M, and Kennedy BK (2008). Quantitative evidence for conserved longevity pathways between divergent eukaryotic species. Genome Res 18(4): 564–570. 10.1101/gr.074724.107
- Paukštyt? J, López Cabezas RM, Feng Y, Tong K, Schnyder D, Elomaa E, Gregorova P, Doudin M, Särkkä M, Sarameri J, Lippi A, Vihinen H, Juutila J, Nieminen A, Törönen P, Holm L, Jokitalo E, Krisko A, Huiskonen J, Sarin LP, Hietakangas V, Picotti P, Barral Y, and Saarikangas J (2023). Global analysis of aging-related protein structural changes uncovers enzyme-polymerization-based control of longevity. Mol Cell 83(18): 3360-3376.e11. 10.1016/j.molcel.2023.08.015
- Okada N, Oshima K, Iwasaki Y, Maruko A, Matsumura K, Iioka E, Vu TD, Fujitsuka N, Nishi A, Sugiyama A, Nishiyama M, Kaneko A, Mizoguchi K, Yamamoto M, and Nishimura S (2021). Intron retention as a new pre-symptomatic marker of aging and its recovery to the normal state by a traditional Japanese multi-herbal medicine. Gene 794: 145752. 10.1016/j.gene.2021.145752
- Vu TD, Ito N, Oshima K, Maruko A, Nishi A, Mizoguchi K, Odaguchi H, Kobayashi Y, and Okada N (2022). Intron retention is a stress response in sensor genes and is restored by Japanese herbal medicines: A basis for future clinical applications. Gene 830: 146496. 10.1016/j.gene.2022.146496
- Yao J, Ding D, Li X, Shen T, Fu H, Zhong H, Wei G, and Ni T (2020). Prevalent intron retention fine-tunes gene expression and contributes to cellular senescence. Aging Cell 19(12): e13276. 10.1111/acel.13276
- Pabis K, Barardo D, Selvarajoo K, Gruber J, and Kennedy BK (2023). A concerted increase in readthrough and intron retention drives transposon expression during aging and senescence. Elife 12: RP87811. 10.7554/eLife.87811.1
- Dvinge H, and Bradley RK (2015). Widespread intron retention diversifies most cancer transcriptomes. Genome Med 7(1): 45. 10.1186/s13073-015-0168-9
- Ni T, Yang W, Han M, Zhang Y, Shen T, Nie H, Zhou Z, Dai Y, Yang Y, Liu P, Cui K, Zeng Z, Tian Y, Zhou B, Wei G, Zhao K, Peng W, and Zhu J (2016). Global intron retention mediated gene regulation during CD4+ T cell activation. Nucleic Acids Res 44(14): 6817–6829. 10.1093/nar/gkw591
- Green ID, Pinello N, Song R, Lee Q, Halstead JM, Kwok CT, Wong ACH, Nair SS, Clark SJ, Roediger B, Schmitz U, Larance M, Hayashi R, Rasko JEJ, and Wong JJ (2020). Macrophage development and activation involve coordinated intron retention in key inflammatory regulators. Nucleic Acids Res 48(12): 6513–6529. 10.1093/nar/gkaa435
- Ziff OJ, Taha DM, Crerar H, Clarke BE, Chakrabarti AM, Kelly G, Neeves J, Tyzack GE, Luscombe NM, and Patani R (2021). Reactive astrocytes in ALS display diminished intron retention. Nucleic Acids Res 49(6): 3168–3184. 10.1093/nar/gkab115
- Steinkraus KA, Kaeberlein M, and Kennedy BK (2008). Replicative Aging in Yeast: The Means to the End. Annu Rev Cell Dev Biol 24: 29–54. 10.1146/annurev.cellbio.23.090506.123509
- Jacob AG, and Smith CWJ (2017). Intron retention as a component of regulated gene expression programs. Hum Genet 136(9): 1043–1057. 10.1007/s00439-017-1791-x
- Lu YY, and Krebber H (2021). Nuclear mRNA Quality Control and Cytoplasmic NMD Are Linked by the Guard Proteins Gbp2 and Hrb1. Int J Mol Sci 22(20): 11275. 10.3390/ijms222011275
- Zenklusen D, Vinciguerra P, Strahm Y, and Stutz F (2001). The Yeast hnRNP-Like Proteins Yra1p and Yra2p Participate in mRNA Export through Interaction with Mex67p. Mol Cell Biol 21(13): 4219–4232. 10.1128/MCB.21.13.4219-4232.2001
- Vinciguerra P, Iglesias N, Camblong J, Zenklusen D, and Stutz F (2005). Perinuclear Mlp proteins downregulate gene expression in response to a defect in mRNA export. EMBO J 24(4): 813–823. 10.1038/sj.emboj.7600527
- Hackmann A, Wu H, Schneider UM, Meyer K, Jung K, and Krebber H (2014). Quality control of spliced mRNAs requires the shuttling SR proteins Gbp2 and Hrb1. Nat Commun 5: 3123. 10.1038/ncomms4123
- Bergeron D, Pal G, Beaulieu YB, Chabot B, and Bachand F (2015). Regulated Intron Retention and Nuclear Pre-mRNA Decay Contribute to PABPN1 Autoregulation. Mol Cell Biol 35(14): 2503–2517. 10.1128/MCB.00070-15
- Li Y, Bor YC, Fitzgerald MP, Lee KS, Rekosh D, and Hammarskjold ML (2016). An NXF1 mRNA with a retained intron is expressed in hippocampal and neocortical neurons and is translated into a protein that functions as an Nxf1 cofactor. Mol Biol Cell 27(24): 3903–3912. 10.1091/mbc.E16-07-0515
- Pimentel H, Parra M, Gee SL, Mohandas N, Pachter L, and Conboy JG (2016). A dynamic intron retention program enriched in RNA processing genes regulates gene expression during terminal erythropoiesis. Nucleic Acids Res 44(2): 838–851. 10.1093/nar/gkv1168
- Luisier R, Tyzack GE, Hall CE, Mitchell JS, Devine H, Taha DM, Malik B, Meyer I, Greensmith L, Newcombe J, Ule J, Luscombe NM, and Patani R (2018). Intron retention and nuclear loss of SFPQ are molecular hallmarks of ALS. Nat Commun 9(1): 2010. 10.1038/s41467-018-04373-8
- Ullrich S, and Guigó R (2020). Dynamic changes in intron retention are tightly associated with regulation of splicing factors and proliferative activity during B-cell development. Nucleic Acids Res 48(3): 1327–1340. 10.1093/nar/gkz1180
- Vernace VA, Schmidt-Glenewinkel T, and Figueiredo-Pereira ME (2007). Aging and regulated protein degradation: who has the UPPer hand? Aging Cell 6(5): 599–606. 10.1111/j.1474-9726.2007.00329.x
- Kaushik S, and Cuervo AM (2015). Proteostasis and aging. Nat Med 21(12): 1406–1415. 10.1038/nm.4001
- Sampaio-Marques B, and Ludovico P (2018). Linking cellular proteostasis to yeast longevity. FEMS Yeast Res 18(5): foy043. 10.1093/femsyr/foy043
- Kruegel U, Robison B, Dange T, Kahlert G, Delaney JR, Kotireddy S, Tsuchiya M, Tsuchiyama S, Murakami CJ, Schleit J, Sutphin G, Carr D, Tar K, Dittmar G, Kaeberlein M, Kennedy BK, and Schmidt M (2011). Elevated Proteasome Capacity Extends Replicative Lifespan in Saccharomyces cerevisiae. PLoS Genet 7(9): e1002253. 10.1371/journal.pgen.1002253
- Townes FW, Carr K, and Miller JW (2020). Identifying longevity associated genes by integrating gene expression and curated annotations. PLoS Comput Biol 16(11): e1008429. 10.1371/journal.pcbi.1008429
- Rodríguez-Navarro S, Sträβer K, and Hurt E (2002). An intron in the YRA1 gene is required to control Yra1 protein expression and mRNA export in yeast. EMBO Rep 3(5): 438–442. 10.1093/embo-reports/kvf091
- Mayor S, and Riezman H (2004). Sorting GPI-anchored proteins. Nat Rev Mol Cell Biol 5(2): 110–120. 10.1038/nrm1309
- Matecic M, Smith DL, Pan X, Maqani N, Bekiranov S, Boeke JD, and Smith JS (2010). A Microarray-Based Genetic Screen for Yeast Chronological Aging Factors. PLoS Genet 6(4): e1000921. 10.1371/journal.pgen.1000921
- Beaupere C, Dinatto L, Wasko BM, Chen RB, VanValkenburg L, Kiflezghi MG, Lee MB, Promislow DEL, Dang W, Kaeberlein M, and Labunskyy VM (2018). Genetic screen identifies adaptive aneuploidy as a key mediator of ER stress resistance in yeast. Proc Natl Acad Sci USA 115(38): 9586–9591. 10.1073/pnas.1804264115
- Brown MK, and Naidoo N (2012). The endoplasmic reticulum stress response in aging and age-related diseases. Front Physiol 3: 263. 10.3389/fphys.2012.00263
- Chadwick SR, and Lajoie P (2019). Endoplasmic reticulum stress coping mechanisms and lifespan regulation in health and diseases. Front Cell Dev Biol 7: 84. 10.3389/fcell.2019.00084
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29(1): 15–21. 10.1093/bioinformatics/bts635
- Robinson MD, McCarthy DJ, and Smyth GK (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1): 139–140. 10.1093/bioinformatics/btp616
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, and Mesirov JP (2005). Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102(43): 15545–15550. 10.1073/pnas.0506580102
- Audic S, and Claverie JM (1997). The Significance of Digital Gene Expression Profiles. Genome Res 7(10): 986–995. 10.1101/gr.7.10.986
- Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12): 550. 10.1186/s13059-014-0550-8
- Huang DW, Sherman BT, and Lempicki RA (2008). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4(1): 44–57. 10.1038/nprot.2008.211
- Kurtenbach S, and Harbour JW (2019). SparK: A Publication-quality NGS Visualization Tool. bioRxiv 845529. 10.1101/845529
–
SUPPLEMENTAL INFORMATION
![]() Download Supplemental Information
Download Supplemental Information
AUTHOR CONTRIBUTIONS
A.d.O.F.d.V. conceived the study. J.G.M. performed most analyses. L.F.D.l.C.G. conducted the GO analysis. J.G.M., A.d.O.F.d.V. and C.Q.S. prepared the first draft of the manuscript with contributions from L.F.D.l.C.G. A.d.O.F.d.V. and C.Q.S. supervised the work. J.M.G.M. supervised the work and provided intellectual and strategic input to the analyses and manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The study was supported by Mexico´s National Council of Humanities, Sciences and Technolo-gies (CONAHCYT).
COPYRIGHT
© 2024

Replicative aging in yeast involves dynamic intron retention patterns associated with mRNA processing/export and protein ubiquitination by Gómez-Montalvo et al. is licensed under a Creative Commons Attribution 4.0 International License.