Research Articles:
Microbial Cell, Vol. 11, No. 1, pp. 106 - 115; doi: 10.15698/mic2024.04.819
The effect of multiple sclerosis therapy on gut microbiota dysbiosis: a longitudinal prospective study
1 Department of Neurosciences, Faculty of Medicine, Iuliu Hat,ieganu University of Medicine and Pharmacy, 400012, Cluj Napoca, Romania. 2 Neurology Department, Cluj Emergency County Hospital, 400012, ClujNapoca, Romania. 3 Faculty of Medicine, Iuliu Hat,ieganu University of Medicine and Pharmacy, 400012, Cluj Napoca, Romania.
Keywords: multiple sclerosis, demyelinating autoimmune disorders, gut microbiota, microbiome, brain gut axis, immunomodulation, immunoglobulin Y.
Abbreviations:
CNS - central nervous system,
EAE - experimental autoimmune encephalomyelitis,
DMT - disease-modifying therapy,
GALT - gut-associated lymphoid tissue,
GI - gastrointestinal,
HC - healthy conrol,
MS - multiple sclerosis,
PwMS - people with MS,
SCFA - short-chain fatty acid.
Received originally: 23/08/2023 Received in revised form: 27/02/2024
Accepted: 01/03/2024
Published: 06/04/2024
Correspondence:
Vitalie Vacaras, vacaras.umfcluj@yahoo.com
Conflict of interest statement: The authors declare no conflict of interest.
Please cite this article as: Andreea-Cristina Paraschiv, Vitalie Vacaras, Cristina Nistor, Cristiana Vacaras, Stefan Strilciuc, Dafin F Muresanu (2024). The effect of multiple sclerosis therapy on gut microbiota dysbiosis: a longitudinal prospective study. Microbial Cell 11: 106-115. doi: 10.15698/mic2024.03.819
Abstract
Gut microbiota has complex immune functions, related to different pathologies, including multiple sclerosis (MS). This study evaluated the influence of treatments on gut microbiota in people with MS (PwMS). The research comprised 60 participants, including 39 PwMS and 21 healthy controls (HC). Among the PwMS, 20 were prescribed a disease-modifying therapy (DMT), either interferon beta1a or teriflunomide, while 19 received a combination of classical DMT and an immunoglobulin Y (IgY) supplement. For each participant, two sets of gut samples were collected: one at the study’s outset and another after two months. Alpha and beta diversity analyses revealed no significant differences between groups. In comparison to the HC, the MS group exhibited an increase in Prevotella stercorea and a decrease in Faecalibacterium prausnitzii. Following treatment, individuals with MS showed enrichment in Lachnospiraceae and Streptococcus. The second sample, compared to the first one, demonstrated an increase in Bifidobacterium angulatum and a decrease in Oscillospira for individuals with MS. Gut microbiota diversity in PwMS is not significantly different to HC. However, specific taxonomic changes indicate the presence of a dysbiosis state. The use of DMTs and immunoglobulin Y supplements may contribute to alterations in microbial composition, potentially leading to the restoration of a healthier microbiome.
INTRODUCTION
Multiple sclerosis (MS), a chronic demyelinating disease affecting the Central Nervous System (CNS), stands as the leading cause of non-traumatic neurological disability among young individuals, with a prevalence surpassing two million cases globally and a higher incidence in females 1. Its pathophysiology involves disruptions in the blood-brain barrier, inflammation, demyelination, and axonal degeneration, resulting in both inflammatory and progressive stages accompanied by diverse neurological manifestations. The symptoms of MS can vary widely, including clinically isolated syndrome (CIS), relapsing-remitting form (RRMS), primary progressive (PPMS), and secondary progressive MS (SPMS) 2. The disease’s etiology is intricate and not entirely comprehended, involving numerous environmental factors acting upon genetic susceptibility 3. Several studies have highlighted the role of the microbiota in immune system maturation, implicating it in various pathologies, including MS 4.
–
The human gut microbiota constitutes a complex biological community in the gastrointestinal (GI) tract, comprising approximately 100 trillion microorganisms exhibiting commensal, symbiotic, and pathogenic characteristics 4. The term “microbiome” refers to the microbial genomes within our body 3. Bacteria are classified taxonomically into various hierarchical levels, including phyla, classes, orders, families, genera, and species, with Firmicutes and Bacteroidetes as prominent phyla. Early-life factors such as delivery method, infant feeding, weaning period, lifestyle, and dietary choices shape the primary microbiota’s composition. Subsequently, after the age of 2, its composition remains relatively stable, with minor changes primarily influenced by environmental exposure 5. Microbes play a crucial role in host physiology, impacting the gut’s motility and permeability, epithelial functions, vitamin production, nutrient metabolism, and prevention of pathogen colonization 4.
–
The gut microbiota is significantly influenced by external factors, such as dietary and lifestyle habits. Consequently, defining a typical healthy microbial ecosystem becomes challenging 6. However, to avoid the onset of diseases and support healthy metabolic and immune functions, it’s crucial for microbial organisms to maintain equilibrium. This optimal microbiota varies from individual to individual 5. A healthy microbiota is generally associated with extensive biodiversity, providing resistance to different stressors and an improved capability for infection clearance and recuperative processes. While dietary habits can quickly alter composition, long-term practices have a more significant impact on the microbiome 7. Dysbiosis, conversely, signifies an imbalance in bacterial composition, leading to disruptions in normal microbial functions, and is implicated in the pathogenesis of various autoimmune disorders, including MS. Inflammation, increased toxins, impaired intestinal permeability, and complex pathological pathways in disease progression are all attributed to dysbiosis 3.
–
Studies have revealed that germ-free rodents do not develop experimental autoimmune encephalomyelitis (EAE), and the severity of EAE can be reduced with antibiotics. These findings have sparked speculation regarding the specific mechanism through which the microbiota influences CNS autoimmunity 4. The gut microbiota-brain axis concept refers to interactions between the GI tract and the CNS through various mechanisms. One pathway involves information exchange via the autonomic nervous system and the vagus nerve. Additionally, stress-induced endocrine activation leads to increased cortisol release by the hypothalamic-pituitary-adrenal axis, potentially influencing the microbiota. The immunoregulation mechanism encompasses immune system development and maturation in the GI tract, T-cell differentiation in gut-associated lymphoid tissue (GALT), and the release of cytokines and lymphocytes. Moreover, microbes produce different neurotransmitters, such as gamma amino acid and dopamine, and the intestinal barrier’s permeability leads to various metabolites (lipopolysaccharide, peptidoglycan) and short-chain fatty acids (SCFAs) that interact with the CNS 8.
–
Numerous studies have established a connection between the gut microbiota and neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and multiple sclerosis. This link is attributed to the microbiome’s involvement in immunoregulatory pathways, which can either protect against or contribute to pathogenesis 9. PwMS have a distinctive gut microbiota composition compared to HC, indicating a pro-inflammatory state. Taxonomic changes in the MS population are inconsistent across some studies. Individuals with inactive RRMS exhibit more Mathanobrevibacter and Akkermansia than HC, with a similar diversity. Conversely, those with an active form of the disease show reduced microbial diversity, coupled with enriched Pseudomonas, Blautia, Dorea, and Haemophilus 10.
–
DMTs encompass specific immunomodulatory drugs designed to decrease clinical and radiological disease activity 4. Based on their efficacy, they are categorized into moderate-efficacy DMTs (interferon beta-IFNβ, teriflunomide-TER, glatiramer acetate-GA, dimethyl fumarate-DMF) and high-efficacy DMTs (natalizumab, anti-CD20 antibodies, cladribine, sphingosine 1 phosphate receptor modulators) 11.
Literature provides evidence that DMTs have an impact on the gut microbiota composition in MS. In a study evaluating the effect of two DMTs on MS microbiota, glatiramer acetate and dimethyl fumarate were associated with a decrease in the Firmicutes and Fusobacteria phyla, a reduction in Lachnospiraceae family, a decrease in Clostridiales, and an increase in the Bacteroidetes phylum. However, these changes did not result in major alterations in composition. One of the functional pathways influenced by these treatments was the metabolism of retinol (vitamin A), acting as a modulator of immunity, particularly the balance between Th1 and Th2, Treg (regulatory T cells), and Th17 cells in GALT. The second metabolic pathway influenced by these therapies involved methane, with observations indicating an elevation in methane-producing bacterial populations in MS patients relative to control groups 12. Another study investigated the impact of four different DMTs on MS microbiota, including ocrelizumab (anti-CD20), dimethyl fumarate, fingolimod, and natalizumab. They found no effect on overall diversity, but anti-CD20 led to increased Faecalibacterium prausnitzii, dimethyl fumarate increased Roseburia intestinalis, both reducing bacteria involved in butyrate metabolism. Fingolimod and natalizumab increased Ruminococcaceae 13.
–
Y antibodies (IgY) are the predominant serum immunoglobulins found in birds, reptiles, and amphibians, serving as counterparts to human IgG and IgE. In comparison to IgG, IgY exhibits increased hydrophobicity and reduced flexibility, resulting in enhanced resistance to proteolytic fragmentation14. IgY binds to antigens, hindering bacterial or viral replication, and aids in eliminating fragments within the gastrointestinal tract 15. In the context of human applications, immunoglobulins Y derived from chicken egg yolk have been extensively investigated for various therapeutic and prophylactic purposes. These include applications in antibacterial, antiviral, antifungal, antitumoral activities, as well as infection diagnostics 14.
–
Our hypothesis posits that the human gut microbiota undergoes significant alterations in individuals with MS, and that specific MS treatments and IgY supplements have the potential to influence and restore the dysbiotic state.
–
The objective of the present study is to characterize the microbiota of individuals with MS in our region and to evaluate the changes in diversity and taxonomy that occur over time and following different therapeutic interventions, including IFN, TER and IgY supplements. To underscore the impact of treatment, we analyzed the microbiota’s characteristics at baseline, prior to any treatment, compared it to the HC group, and delineated various alterations observed between the groups and within the two samples.
RESULTS
The study employed alpha and beta diversity analyses to examine differences in bacterial composition between groups. The 16S analysis provided information on the frequency and relative abundance of identified species, allowing for the analysis of various taxonomic differences between cohorts.
Baseline cohort’s characteristics and microbiota profile
The median age of the RRMS group was 36±10 years, consisting of 25 females and 14 males. Only four participants had received a form of DMT in the past, but not in the previous year, while the rest were treatment naïve. Details regarding disease characteristics are provided in Table 1. The HC group exhibited similar demographic features, with a median age of 28±9.8 years and a female predominance of 61%.
Table 1. Baseline characteristics of PwMS.
|
Characteristics |
PwMS (n=39) |
|---|---|
|
MS type, n (%) |
|
|
RRMS |
39 (100%) |
|
EDSS score, median (range) |
1.5 (0-5) |
|
Confirmed disease duration, mean (SD) (y) |
1.94 (3.0) |
|
Treatment, n (%) |
|
|
Teriflunomide |
9 (23%) |
|
Interferon beta1a |
11 (28.2%) |
|
Teriflunomide+ immunoglobulins Y |
10 (25.6%) |
|
Interferon beta+ immunoglobulins Y |
9 (23%) |
MS=multiple sclerosis, PwMS=people with MS, n=number of patients, SD=standard deviation, y=years, RRMS= relapsing-remitting MS, EDSS=Expanded Disability Status Scale.
At baseline, we detected 51 different phylum OTUs (operational taxonomic units) in the MS cohort, including Firmicutes (49.49%), Bacteroidetes (34.09%), Bacteria (6.48%), and Actinobacteria (5.15%). At the species level, we identified 1512 OTUs, such as Prevotella copri (11.86%), Bacteroides (9.17%), Faecalibacterium prausnitzii (6.1%), and Blautia (4.4%).
When assessing alpha diversity in MS samples before treatment, no significant differences were observed between G(MS) and G(HC) using Chao (p=0.14), Shannon (p=0.81), or Simpson indexes (p=0.62). Please refer to Supplemental Table S1 (supplementary data) for all p values related to microbial diversity comparisons.
In comparison to the HC group, untreated PwMS exhibited an elevated relative abundance of Prevotella sterocorea (p=0.01) and Bacteroidales (p=0.003). Additionally, they showed a reduction in Faecalibacterium prausnitzii (p=0.004), Bifidobacterium adolescentis (p=0.002), Bifidobacterium angulatum (p=0.004), Coprococcus (p=0.02), Lachnospiraceae species (p=0.03).
Longitudinal changes in gut microbiota
After a two-month treatment period, there were no observed changes in alpha (p=0.67) or beta diversity (p=0.23) between the PwMS and the HC group (refer to Table S1).
In comparison to HC, PwMS who underwent treatment showed an increased relative abundance of Prevotella stercorea (p=0.02), Streptococcus (p=0.04), and Lachnospiraceae (p=0.02), along with a decreased relative abundance of Ruminococcus (p=0.02) and Helicobacter pylori (p=0.01).
The comparison between Sample 2 and Sample 1 within each group revealed no significant changes in alpha or beta diversity, as detailed in Table S1.
Compared to the baseline, treated PwMS exhibited elevated levels of Bifidobacterium angulatum (p=0.01) and reduced levels of Oscillospira (p=0.01), Anaerotruncus (p=0.03), Rumen bacteria (p=0.04), and Sporobacter (p=0.02) (Fig. S1, Fig. S2, Fig. S3).
The second sample from the group receiving DMT (G(DMT)) highlighted increased levels of Bifidobacterium angulatum (p=0.04) and decreased relative abundances of Oscillospira (p=0.004), Butyricimonas (p=0.04), Lachnospiraceae (p=0.03), and Sporobacter (p=0.02), when compared to the baseline (refer to Fig. S4).
The effect of specific treatments on gut microbiota
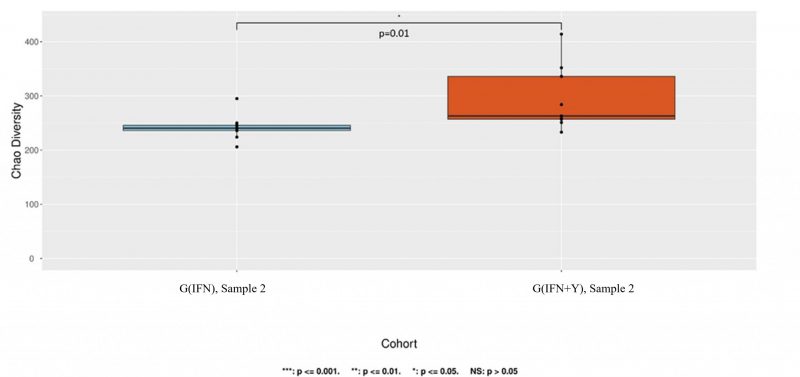
We examined whether each specific therapy had an impact on overall microbial diversity. The alpha diversity between the group treated with teriflunomide (G(TER)) and the group receiving a combination of teriflunomide and IgY supplement (G(TER+Y)) was not altered for the second sample (p=0.55). However, a statistically significant difference in alpha diversity for the second sample was observed between subgroups treated with interferon beta1a (G(IFN)) and those receiving a combination of interferon beta1a and IgY supplement (G(IFN+Y)) (p=0.01, Chao index) (refer to Fig. 1).
–
As we observed this difference for the first sample as well (Chao index, p=0.00097), we investigated potential environmental or lifestyle confounders that might have influenced the results. We analyzed several variables obtained from questionnaires using the Chi-square test for these two specific groups with significant diversity alterations. A significant difference between the two groups was found in terms of smoking (p=0.02) and the method of birth (natural or caesarean, p=0.04). However, no significant differences were noted for living environment (urban or rural, p=0.76), weight (overweight-obese and normal weight, p=0.42), or being breastfed (only one patient was not breastfed).
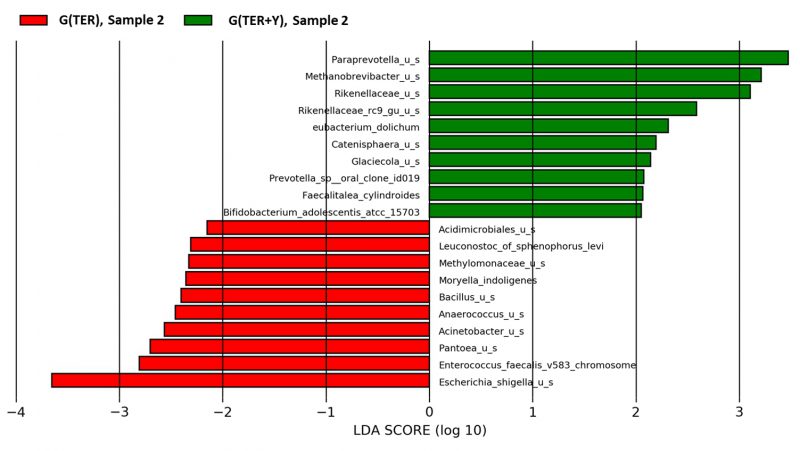
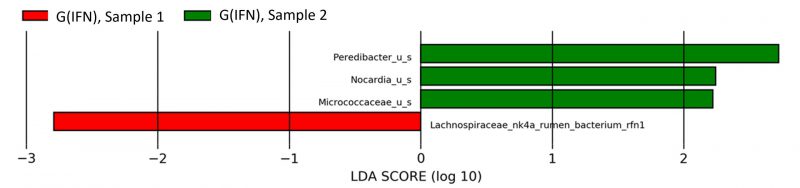
When compared to G(IFN+Y), the subgroup G(IFN) exhibited increased relative abundance of Collinsella (p=0.02) and decreased Lachnospiraceae rumen (p=0.01) and Bilophila (p=0.04) (Fig. 2) after treatment. When compared to G(TER+Y), G(TER) had a higher relative abundance of Enterococcus faecalis (p=0.03) and a lower relative abundance of Mathanobrevibacter (p=0.04) and Paraprevotella (p=0.03) after two months of treatment (Fig. 3).
–
–
Longitudinal analyses of the second sample compared to the first one revealed no significant overall diversity changes (Table S1, Fig. S5).
–
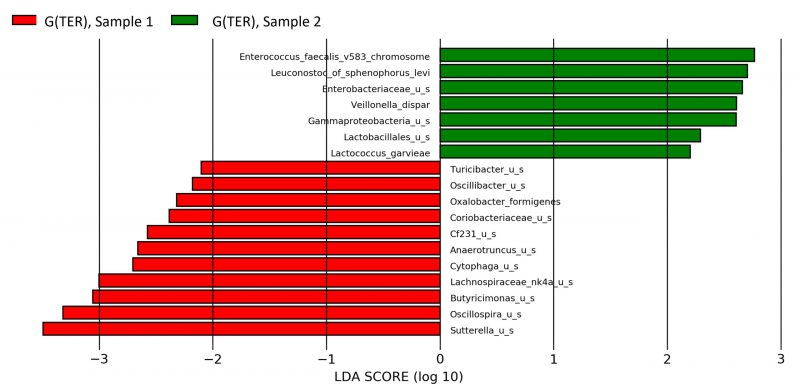
A reduction of Lachnospiraceae (p=0.04) and an increase of Peredibacter (p=0.0008) were encountered in the second sample of G(IFN) compared to the first one, as outlined in Fig. 4. Teriflunomide treatment was associated with increased Enterococcus faecalis (p=0.02), Enterobacteriaceae (p=0.02), and Gammaproteobacteria (p=0.03) and reduced Sutterella (p=0.02), Oscillospira (p=0.04), and Butyricimonas (p=0.01) in the G(TER) group, changes emphasized in Fig. 5.
–
For the second sample, the subgroup G(IFN+Y) showed reduced Haemophilus species (p=0.02) than for the first one. The group G(TER+Y) presented no significant taxonomic differences between the two samples, all p>0.05.
–
DISCUSSION
Microbiota particularities in treated and untreated PwMS
We noted no major difference in alpha or beta microbiota diversity between untreated or treated PwMS and controls. This is similar to most studies, as the core microbiome is established in early childhood, and environmental factors have minimal influence thereafter 16, 17.
Our results stated that the baseline MS microbiota was associated with significant increase in Prevotella stercorea and reduced Faecalibacterium prausnitzii, Bifidobacterium, and Lachnospiraceae compared to the HC group. For treated PwMS, there was an increase in Prevotella stercorea, Lachnospiraceae and a decrease in Ruminococcus species.
We encountered a variety of contradictory findings in the literature regarding the bacterial composition of PwMS. In the microbiota of most healthy individuals, the dominant phyla are Bacteroidetes and Firmicutes, together accounting for 90% of the total microbial composition. Nevertheless, in conditions of disease, there is a noticeable increase in other microorganisms, including Proteobacteria, Verrucomicrobia, and Actinobacteria 6. Within the Firmicutes phylum, there is often an increase in Clostridium perfringens, recognized as a potential trigger for the pathological process, alongside a decrease in Roseburia, Lachnospiraceae, and Ruminococcaceae in individuals with MS, compared to HC. A reduction in butyrate-producing bacteria, such as Firmicutes, may compromise the intestinal barrier. Reduced in MS, Bacteroidetes is involved in propionate metabolism, a SCFA with anti-inflammatory properties that stimulate Treg cells, and it produces lipid 654, a lipopeptide that regulates the innate immune system. PSA, an immunoregulatory polysaccharide produced by Bacteroides fragilis, has also been reported to be diminished in the majority of MS studies 18. Faecalibacterium prausnitzii, known for its anti-inflammatory properties, was found to be reduced in individuals with inflammatory diseases 16. In the scientific literature, Prevotella is consistently reported to show a reduced relative abundance in PwMS; however, the majority of the research concentrates on Prevotella copri. Notably, the relative abundance of Prevotella copri returns to baseline levels following treatment specific to MS. Most studies focused on Prevotella were heterogeneous regarding the DMTs, and they found an inverse correlation between inflammation and Prevotella abundance 10. Additionally, rodent trials provided evidence of Prevotella histicola causing a shift in microbial composition and suppressing EAE10. Our cohort was associated at baseline with a reduction of Faecalibacterium prausnitzii and an increase in Lachnospiraceae after one year of treatment, both bacteria with crucial roles in SCFA metabolism and a reduction in epithelial permeability by increasing tight junction proteins 5.
By examining the longitudinal changes in our study, it becomes evident that individuals with MS experienced an increase in Bifidobacterium angulatum after one year of treatment compared to baseline. Bifidobacterium angulatum is considered to be involved in the differentiation of Treg cells1. Various probiotics, such as a combination of Lactobacillus and other Bifidobacterium species, have demonstrated improvements in MS clinical outcomes in several studies 19, 20.
Clostridium perfringens‘ Epsilon toxin (ETX) has the ability to breach the blood-brain barrier and bind to oligodendrocytes, making it a likely promoter of demyelination and MS lesions1, 12. Its frequent observation in PwMS suggests a potential contribution to MS pathology and certain DMTs have been shown to inhibit Clostridium perfringens 4. In our longitudinal research, we found no significant differences regarding this species in treated MS patients.
–
Microbial diversity and taxonomy changes associated with specific treatments
Our MS cohort was not associated with any significant diversity changes in IFN or TER treated participants, compared to untreated individuals. Compared to baseline, people treated with IFN presented reduced Lachnospiraceae, while for TER treated group, we observed an increased Enterococcus faecalis and reduced Sutterella compared to the first sample.
–
Interferons are cytokines that regulate inflammatory responses to pathogenic agents, including stimuli related to the intestine 11 and they have the capability to modify the properties of the intestinal barrier 4. The IFN family is categorized into distinct types: IFN type I (including IFN-β), II, and III. They influence the immune system through different mechanisms, including the effects on dendritic cells or T-cell signalling 21. Interferon beta-1a inhibits T cell division, blood-brain barrier migration, and other proinflammatory cytokines while inducing Treg and suppressive B cells 2. There is a connection between the gut immune system and the IFN response through two pathways. First, metabolites of commensal microbes travel through systemic circulation and reach the immune system. The second mechanism involves circulating immune cells interacting with the gut microbiota and then migrating to other immune systems in different organs 21. Numerous findings suggest that the microbiota influences IFN signalling 22. Firstly, the expression of IFN is upregulated through direct interactions with either beneficial or pathogenic microbes. Secondly, the microbiota influences IFN through its metabolites, including the impact of SCFAs, derived secondary bile acids signalling, and tryptophan 23. An increase in serum propionic acid, an SCFA promoting Treg cell induction and disease improvement, was found in MS participants treated with IFN 11. All these mechanisms illustrate how the gut microbiota influences IFN signalling.
IFN-β1a modulates interactions between epithelial cells and commensal bacteria and it stabilizes biological barriers by upregulating tight junction proteins 24. In some studies we find increased Prevotella and Sutterella and decreased Sarcina species in IFN-β treated compared to untreated participants, similar to healthy controls 4, 11, 12. In the literature, this possible shift to a healthier microbiota state is considered to be related to the complex IFN mechanism of downregulating proinflammatory cytokines 17. In a study analyzing the microbiota changes associated with IFNβ treatment in MS, there was no overall diversity modification after IFN 17.
Teriflunomide is a frequently prescribed DMT that hinders pyrimidine synthesis by targeting dihydroorotate dehydrogenase. This action inhibits proinflammatory cytokines and T cell activation 2. TER demonstrates a bacteriostatic effect by impeding the in vitro growth of C. perfringens toxins. The epsilon toxin from C. perfringens exhibits tropism for the blood-brain barrier and myelin, potentially contributing to the development of new lesions in MS 24, 25. Furthermore, TER enhances the population of protective CD39+ Treg cells in the GALT tissue of mice. In murine models, TER reduces antigen-presenting cells in Peyer patches 26.
Both interferon and teriflunomide treatments in our cohort were associated with a reduction in Lachnospiraceae species, microorganisms that have been implicated in impairing oligodendrocyte differentiation in cultured cells and reducing myelination capacity in mice 12. In a study performed on a larger cohort of 168 MS cases, two commonly used disease-modifying treatments, glatiramer acetate and dimethyl fumarate, were also associated with a decreased relative abundance of Lachnospiraceae 12. The same mentioned study 12 noted a significant decrease in Sutterella species in participants treated with glatiramer acetate compared to the naïve patients. In our cohort, a similar decrease in relative abundance was observed in teriflunomide-treated patients compared to their baseline microbiome profile.
However, in our cohorts, we observed a notable difference in alpha diversity between the interferon group and the combination of interferon plus immunoglobulin Y group. Although the p-value was significant (p=0.01), this significance was observed only for the Chao index and not for the Shannon or Simpson parameters. We also noted a difference in diversity in the first sample. Therefore, attributing this diversity change to the IgY supplement therapy may be challenging, and it could be due to sample size or chance. Furthermore, we conducted an analysis to assess the impact of potential confounding factors, which are known to influence the microbiome 1, based on the data collected. The findings revealed notable distinctions between the interferon group and the interferon plus IgY supplement group concerning smoking status (p=0.02) and birth method (p=0.04). These confounders could potentially contribute to the observed variations in diversity.
In our cohort, a decrease in Haemophilus species was observed in individuals receiving combined treatment with interferon and IgY, compared to baseline levels. Interestingly, a recent study investigating microbial alterations and symptoms of schizophrenia revealed a positive correlation between Haemophilus abundance and negative psychiatric manifestations 27. This study suggests a potential adverse role of Haemophilus species in neuropsychiatric disorders. Therefore, the observed reduction in Haemophilus abundance following IFN and IgY treatment may indicate a shift towards a healthier microbiota state.
Immunoglobulins Y oral supplementation is a well-researched method for the prevention and treatment of several bacterial, viral, or fungal infections in animals and humans 28. Studies have revealed a potential connection between immunoglobulins Y supplements and the gut microbiome. Although IgY supplementation is not a probiotic or a prebiotic on its own, it can be used as a nutraceutical ingredient in various probiotic combinations to enhance its effectiveness. The combination of a probiotic and IgY supplement has been shown to have an increased beneficial effect compared to each isolated treatment 29. While IgY supplements are being tested for various autoimmune diseases, such as psoriasis, where they reduced disease severity in animal models 30, oral IgY supplementation has not been studied in the microbiome of the MS population. Future research directions may involve exploring its effects on different autoimmune or degenerative pathologies in humans.
Our findings suggest that specific MS treatments and oral IgY supplementation are associated with changes in the MS microbiome.
–
Strengths and limitations
The current study delved into the longitudinal changes in the microbiome of individuals with MS following exposure to various treatments. It takes into consideration not only interferon treatment, but also teriflunomide, as an oral first line therapy less studied in relation to microbiome. Additionally, we introduced a potentially influential factor, oral immunoglobulin Y therapy, which has been less extensively studied in the MS population.
A limitation of the current study is the relatively small cohort sizes and the presence of numerous dietary or environmental factors that can interfere with the gut microbiome, making it challenging to assert causality. Furthermore, the non-randomized nature of patient selection, based on disease profile, comorbidities, lifestyle conditions, and personal preferences, could introduce bias into the results. For instance, some patients specifically requested oral treatment instead of intramuscular, potentially influencing the outcomes. The effect of the IgY supplement may be subject to bias as the second sample was collected two months after the first one, even though the IgY treatment was administered for only one month, as specified by the manufacturer. The choice of a two-month interval for the two samples was influenced by timepoints in other studies and internal administrative considerations. Larger trials are necessary to confirm the generalizability of these results.
The comparisons between G(DMT) vs G(DMT+Y) (sample 2) and G(MS) vs G(HC) (sample 2) are potentially biased, and causality cannot be definitively established.
–
Conclusions
MS has been linked to notable changes in specific microbial taxa rather than substantial shifts in community composition. Untreated PwMS displayed elevated levels of Prevotella sterocorea and decreased levels of Faecalibacterium prausnitzii, while treated MS patients exhibited increased levels of Lachnospiracea compared to healthy controls. After two months of interferon treatment, a reduction in Lachnospiraceae was observed in the second sample compared to the first one, while teriflunomide was associated with increased Enterococcus faecalis and Gammaproteobacteria.
The gut microbiota, a complex ecosystem with various immune functions, plays a significant role in MS, and its response to different treatments is highlighted in this study. In clinical practice, maintaining a healthy microbiota is crucial for individuals with MS.
MATERIALS AND METHODS
Subjects and sample collection
A total of 60 individuals, consisting of 39 PwMS and 21 HC, participated in this prospective, longitudinal, analytical, observational, and case-control study. The data were collected from the Neurology Department of the Emergency County Hospital Cluj-Napoca between January 2019 and May 2020. Ethical authorization (number 2394/28.01.2020) was obtained, and each participant provided written informed consent.
The study included adult patients diagnosed with RRMS who met the revised McDonald diagnostic criteria 31. Participants had not received any DMTs for at least one year before the study was initiated and had an Expanded Disability Status Scale (EDSS) score of less than 5. Individuals with a progressive form of the condition were excluded. The HC group comprised healthy adults without known pathologies or chronic treatments, with demographics similar to the MS group. Exclusion criteria for both groups included pregnancy, breastfeeding, acute or chronic moderate to severe gastrointestinal pathologies, and any long-term medical treatments within the previous 6 months that could influence gut microbiota, such as proton-pump inhibitors, antibiotics, non-steroidal anti-inflammatory drugs, antivirals, and probiotics. Only three subjects had mild compensated gastritis, while other chronic diseases (diabetes, arterial hypertension, thyroid dysfunction) were isolated cases and clinically stable.
Throughout the study, participants maintained a varied and stable diet without major restrictions, as declared in our questionnaire. We designed a simple questionnaire for subjects to fill out, providing basic demographic, disease, and dietary information, including dietary habits and any changes during the study data collection period.
Upon enrollment in the study, each participant provided the initial faecal sample (referred to as Sample 1, S1), collected in specific containers following the manufacturer’s guidelines. These samples were stored at -20 degrees Celsius for 24 hours before being transported to the laboratory for analysis. After the initial samples, the MS group underwent treatment with a DMT based on their clinical features, comorbidities, and national medication availability. For recently diagnosed patients, the suitable treatment options were either interferon beta1a, administered intramuscularly at 30 g/0.5 ml once a week, or oral teriflunomide at 14 mg daily. The second sample (Sample 2, S2) was collected under similar conditions, eight weeks after the initial one. A subset of patients consented to receive immunoglobulin Y supplements in addition to the standard DMT. The inclusion of immunoglobulin Y supplements in the treatment of MS patients was categorized as an experimental component of the main study. This classification was based on the fact that patients received approved standard care for MS without any intervention from our project’s perspective, thus maintaining the study’s observational nature. However, certain subjects voluntarily opted to receive the IgY supplement as a part of the experimental project. This experimental design, combining observational and experimental elements, received approval from the same ethics committee, including product authorization and all dosage details.
The IgY supplement is a commercially produced egg yolk-derived blend of IgY targeting various infections, including Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, Enterococcus faecalis, Salmonella Enteritidis, Salmonella Typhimurium, Streptococcus mutans, group B and pneumoniae, Staphylococcus aureus, Proteus, Acinetobacter baumanii, Helicobacter pylori, Clostridium difficile, Candida spp. This supplement is stored in cold conditions (2-8 Celsius grades) in granule form. Participants dissolved 12 grams of granules (equivalent to two dosing spoons), providing 200 mg of specific IgY, in room temperature liquid (water, juice, milk) daily for 30 consecutive days. The IgY supplement was administered concurrently with the onset of the DMT treatment. The treatment groups are outlined in Table 2: group G(DMT) received DMT, group G(DMT+Y) received a combination of DMT and IgY supplement, and group G(HC) consisted of HC with no treatment. The collective group of all PwMS was designated as G(MS).
Table 2. Groups’identification acronyms.
|
Group acronym |
Description |
Number of subjects |
Subgroup |
Description |
Number of subjects |
|---|---|---|---|---|---|
|
G(DMT) |
Disease-modifying therapy |
20 |
G(IFN) |
Interferon beta1a |
11 |
|
|
|
|
G(TER) |
Teriflunomide |
9 |
|
G(DMT+Y) |
Disease-modifying therapy and immunoglobulins Y supplement |
19 |
G(IFN+Y) |
Interferon beta1a and immunoglobulins Y supplement |
9 |
|
|
|
|
G(TER+Y) |
Teriflunomide and immunoglobulins Y supplement |
10 |
|
G(HC) |
Healthy controls |
21 |
|
|
|
|
G(MS) |
All people with MS |
39 |
|
|
|
DMT=disease modifying treatment, IFN=interferon beta1a, TER= teriflunomide,Y=immunoglobulins Y, HC=healthy controls, MS=multiple sclerosis.
The 16S taxonomic sequencing and statistical analysis
DNA extraction was performed on each fecal sample and subsequently processed utilizing the Illumina MiSeq platform. Sequencing of the hypervariable regions (V1-V3, V3-V4, ITS1&ITS2) of the 16S subunit ribosomal RNA gene (16rRNA) was conducted, generating outcomes in operational taxonomic units (OTUs). These OTUs represent microorganisms sharing at least 97% DNA similarity with the laboratory’s database. Subsequent classification of these OTUs into bacterial taxa was based on their relative abundance.
Biological diversity refers to the variability among living organisms in an ecosystem, quantified through mathematical measures such as richness (the number of different species) and evenness (the uniformity of population size). Alpha diversity, denoting diversity within samples, is assessed using Chao for richness and Shannon and Simpson as estimators for richness and evenness. These indices values are associated with species number and an equitable distribution 32. Beta diversity, representing diversity between samples, is gauged by Bray-Curtis and Jaccard indexes, where the distance in their graphical representation reflects differences between samples. Principal Coordinate Analysis (PCoA) figures are generated using relative abundance data. Linear Discriminant Effect Size (LEfSe) highlights differences in relative abundance between the two groups, employing an alpha value of 0.05 and a logarithmic score (LDA) of 2. The displayed bacteria have LDAs between -2.0 and 2.0 and are color-coded in green and red, indicating enrichment or reduction compared to the other group. Stacked bars illustrate the average relative abundance of the top 25 species, and microbial overview is presented using heatmaps.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by Cluj Emergency County Hospital Ethics Committee, code 2394/28.01.2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Due to privacy concerns, the data and other information from this study are only available upon request from the study’s corresponding author.
SUPPLEMENTAL INFORMATION
![]() Download Supplemental Information
Download Supplemental Information
AUTHOR CONTRIBUTIONS
Conceptualization, V.V.; methodology and resources, A-C.P, C.N.; formal analysis, C.N.; data curation, C.V.; writing-original draft preparation, A-C.P, C.N.; writing- review & editing, S.S.; supervision D.F.M. All authors approved the published version of the manuscript.
ACKNOWLEDGMENTS
We acknowledge the contribution of CosmosID laboratory for analyzing our samples and for statistical analysis support. We also appreciate the help of Ioana Pinzaru for a high-quality English revision.
The study was founded by personal means, and the immunoglobulins Y supplements were obtained under financial collaboration with Romvac Company, registry number 593/09.01.2020.
COPYRIGHT
© 2024

The effect of multiple sclerosis therapy on gut microbiota dysbiosis: a longitudinal prospective study by Paraschiv et al. is licensed under a Creative Commons Attribution 4.0 International License.