
Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.

Luminal acetylation of microtubules is not essential for Plasmodium berghei and Toxoplasma gondii survival
Acetylation of α-tubulin at lysine 40 is not essential for cytoskeletal stability in Plasmodium berghei or Toxoplasma gondii, suggesting redundancy and plasticity in microtubule regulation in these parasites.

The dual-site agonist for human M2 muscarinic receptors Iper-8-naphtalimide induces mitochondrial dysfunction in Saccharomyces cerevisiae
S. cerevisiae is a model to study human GPCRs. N-8-Iper, active against glioblastoma via M2 receptor, causes mitochondrial damage in yeast by binding Ste2, highlighting evolutionary conservation of GPCRs.

Integrative Omics reveals changes in the cellular landscape of peroxisome-deficient pex3 yeast cells
To uncover the consequences of peroxisome deficiency, we compared Saccharomyces cerevisiae wild-type with pex3 cells, which lack peroxisomes, employing quantitative proteomics and transcriptomics technologies.

Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
Rebekkah E. Pope1, Patrick Ballmann2, Lisa Whitworth3 and Rolf A. Prade1,*
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
Evelyn Tevere1,a, María G. Mediavilla1,a, Cecilia B. Di Capua1, Marcelo L. Merli1, Carlos Robello2,3, Luisa Berná2,4 and Julia A. Cricco
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.
Sir2 regulates selective autophagy in stationary-phase yeast cells
Ji-In Ryua, Juhye Junga, and Jeong-Yoon Kim
This study establishes Sir2 as a previously unrecognized regulator of selective autophagy during the stationary phase and highlight how cells dynamically control organelle degradation.
Cross-species complementation of bacterial- and eukaryotic-type cardiolipin synthases
Petra Gottier1, Mauro Serricchio1, Rita Vitale2, Angela Corcelli2, and Peter Bütikofer1
This article shows that cardiolipin is crucial for cellular respiration and membrane integrity, with cardiolipin synthase enzymes like TbCLS in Trypanosoma brucei being potential drug targets due to their essential role in survival. The study demonstrates TbCLS’s ability to restore cardiolipin production in yeast, highlighting the specificity and potential co-localization required for cardiolipin synthesis and remodeling, and underscoring the differences between eukaryotic and prokaryotic cardiolipin synthase mechanisms.
Identification of SUMO conjugation sites in the budding yeast proteome
Miguel Esteras1, I-Chun Liu1, Ambrosius P. Snijders2, Adam Jarmuz1 and Luis Aragon1
The authors present a proteomic study that mapped SUMO acceptor lysines in budding yeast, identifying 257 potential conjugation sites, including both known and novel substrates, and providing a significant resource for future research into the functional implications of SUMOylation in yeast.
Ydj1 governs fungal morphogenesis and stress response, and facilitates mitochondrial protein import via Mas1 and Mas2
Jinglin L. Xie2,#, Iryna Bohovych3,#, Erin O.Y. Wong2, Jean-Philippe Lambert4, Anne-Claude Gingras2,4, Oleh Khalimonchuk3,5,6, Leah E. Cowen2 and Michelle D. Leach1,2
The authors descibe the role of the Hsp40 chaperone Ydj1 in Candida albicans, noting its localization to the cytosol and mitochondrial membrane, its necessity for stress responses and filamentation, and its involvement in a protein interaction network related to co-chaperones, filamentation regulators, and mitochondrial processing peptidases, with a particular focus on the impact of Ydj1 on mitochondrial morphology, function, and the import of precursor proteins.
Farnesol inhibits translation to limit growth and filamentation in C. albicans and S. cerevisiae
Nkechi E. Egbe1,2, Tawni O. Dornelles1, Caroline M. Paget1, Lydia M. Castelli1,3 and Mark P. Ashe1
Farnesol, a quorum-sensing molecule, inhibits the switch from yeast to filamentous growth in Candida albicans by impeding translation initiation, differing from fusel alcohols that affect the initiation factor eIF2B, as it disrupts mRNA interaction with the ribosome and prevents preinitiation complex formation.
Cristae architecture is determined by an interplay of the MICOS complex and the F1FO ATP synthase via Mic27 and Mic10
Katharina Eydt1,2, Karen M. Davies3, Christina Behrendt4, Ilka Wittig1,5 and Andreas S. Reichert1,2,4,*
This article investigates the roles of MICOS subunits Mic27 and Mic10, revealing their antagonistic and cooperative interactions in crista junction formation and cristae membrane curvature, and proposes a model where F1FO-ATP synthase is connected to MICOS, influencing CJ formation.
Integrative modules for efficient genome engineering in yeast
Triana Amen1 and Daniel Kaganovich1
The study introduces a set of vectors with integrative modules designed for effective genome integration into standard marker loci of Saccharomyces cerevisiae, enabling precise expression levels using various promoters and demonstrating the capability of stable multi-gene integration, which is useful for tasks like multi-color cellular imaging and metabolic engineering.
The neuroprotective steroid progesterone promotes mitochondrial uncoupling, reduces cytosolic calcium and augments stress resistance in yeast cells
Slaven Stekovic1,*, Christoph Ruckenstuhl1,*, Philipp Royer1, Christof Winkler-Hermaden1, Didac Carmona-Gutierrez1, Kai-Uwe Fröhlich1, Guido Kroemer3-8, and Frank Madeo1,2
Progesterone, known for its role in the reproductive system, also acts as a neurosteroid and has been suggested to aid recovery from traumatic brain injury; a study using yeast models shows that progesterone can protect against apoptosis, reduce oxidative stress and calcium spikes, and increase mitochondrial function, independent of traditional progesterone receptors or calcium transporters.
A simple microfluidic platform to study age-dependent protein abundance and localization changes in Saccharomyces cerevisiae
Margarita Cabrera1,†, Daniele Novarina1, Irina L. Rempel1, Liesbeth M. Veenhoff1, and Michael Chang1
We have developed a user-friendly microfluidic system paired with a genetic approach to enrich and study ageing mother yeast cells, enabling the monitoring of protein abundance and localization changes during the crucial first half of their replicative lifespan, leading to the discovery of novel age-dependent protein behaviors.
Thiol trapping and metabolic redistribution of sulfur metabolites enable cells to overcome cysteine overload
Anup Arunrao Deshpande1,#, Muskan Bhatia1,#, Sunil Laxman2, Anand Kumar Bachhawat1
In this study, researchers investigate the mechanisms for handling cysteine overload using Saccharomyces cerevisiae, finding that overexpressing the high affinity cysteine transporter, YCT1, enables yeast cells to rapidly accumulate high levels of intracellular cysteine. The study demonstrates that cells can manage potentially toxic levels of cysteine by converting it to non-reactive thiol forms and utilizing the metabolic products for cell growth.
New insights in the mode of action of anti-leishmanial drugs by using chemical mutagenesis screens coupled to next-generation sequencing
Arijit Bhattacharya1, Sophia Bigot2, Prasad Kottayil Padmanabhan2, Angana Mukherjee2, Adriano Coelho3, Philippe Leprohon2, Barbara Papadopoulou2 and Marc Ouellette2
In this article, the authors comment on the study “Coupling chemical mutagenesis to next generation sequencing for the identification of drug resistance mutations in Leishmania” by Bhattacharya et al. (Nat Commun, 2019), which introduces Mut-seq, a chemical mutagenesis and sequencing approach, to uncover drug resistance mechanisms in Leishmania, revealing links between lipid metabolism genes and miltefosine resistance, and a protein kinase involved in translation conferring paromomycin resistance.
Microfluidic techniques for separation of bacterial cells via taxis
Jyoti P. Gurung1, Murat Gel2,3 and Matthew A. B. Baker1,3
Microfluidic tools, ideal for studying microbial motility due to their control over laminar flows at microscopic scales, enable precise analysis of various taxis behaviors and have advanced applications in synthetic biology, directed evolution, and medical microbiology.
Influence of delivery and feeding mode in oral fungi colonization – a systematic review
Maria Joao Azevedo1,2,3,4, Maria de Lurdes Pereira1,5, Ricardo Araujo2,3,6, Carla Ramalho3,7,8, Egija Zaura4 and Benedita Sampaio-Maia1,2,3
A systematic review of oral fungal colonization in infants found that while breastfeeding did not significantly affect the oral mycobiome, vaginal delivery was associated with higher oral yeast colonization, particularly of Candida albicans.
A holobiont view on thrombosis: unravelling the microbiota’s influence on arterial thrombus growth
Giulia Pontarollo1, Klytaimnistra Kiouptsi1 and Christoph Reinhardt1,2
In this article, the authors comment on the study “The microbiota promotes arterial thrombosis in low-density lipoprotein receptor-deficient mice” by Kiouptsi et al. (mBio, 2019) that showed that commensal microbiota, intricately linked to host physiology, may influence cardiovascular disease, as shown by studies using germ-free atherosclerosis-prone mice to examine how microbial presence and diet affect arterial thrombosis and lesion development.
The role of Lactobacillus species in the control of Candida via biotrophic interactions
Isabella Zangl1, Ildiko-Julia Pap2, Christoph Aspöck2 and Christoph Schüller1,3
Microbial communities, including Candida and Lactobacillus species, play a crucial role in human health, particularly in the context of mucosal infections, but our understanding of their interactions and effects is still incomplete due to the variability of species and isolates as well as the complexity of the human host.
Tribal warfare: Commensal Neisseria kill pathogen Neisseria gonorrhoeae using its DNA
Magdalene So1 and Maria A. Rendón1
This article comments on work published by Kim et al (Cell Host Microbe, 2019), which adds a new dimension to the concept of commensal protection. It shows that commensal Neisseria kill the closely related pathogen N. gonorrhoeae through an unexpected mechanism, one that involves genetic competence, DNA methylation state and recombination.
Yet another job for the bacterial ribosome
Andrea Origi1,2, Ana Natriashivili1,2, Lara Knüpffer1, Clara Fehrenbach1, Kärt Denks1,2, Rosella Asti1 and Hans-Georg Koch1
This article comments on work published by Knüpffer et al (mBio, 2019), which revealed the intricate interaction of uL23 with yet another essential player in bacteria, the ATPase SecA, which is best known for its role during post-translational secretion of proteins across the bacterial SecYEG translocon
Gut microbial metabolites in depression: understanding the biochemical mechanisms
Giorgia Caspani1, Sidney Kennedy2-5, Jane A. Foster6 and Jonathan Swann1
This article shows how the gut microbiota contributes to the pathophysiology of depression and examines the mechanisms by which microbially-derived molecules may influence depressive behavior, highlighting the potential of dietary interventions as novel therapeutic strategies.
The multiple functions of the numerous Chlamydia trachomatis secreted proteins: the tip of the iceberg
Joana N. Bugalhão1 and Luís Jaime Mota1
CThis article shows an in-depth review on the current knowledge and outstanding questions about secreted proteins from Chlamydia trachomatis, detailing their roles in host cell interaction and immune response evasion.
The emerging role of complex modifications of tRNALysUUU in signaling pathways
Patrick C. Thiaville1,2,3,4 and Valérie de Crécy-Lagard2,4
This comment discusses the article “Loss of wobble uridine modification in tRNA anticodons interferes with TOR pathway signaling” by Scheidt et al (Microbial Cell, 2014).
Only functional localization is faithful localization
Roland Lill1,2,3
This article comments on work published by Peleh et al. (Microbial Cell 2014), which analyzes the localization of Dre2 in Saccharomyces cerevisiae.
One cell, one love: a journal for microbial research
Didac Carmona-Gutierrez1, Guido Kroemer2-6 and Frank Madeo1
In this inaugural article of Microbial Cell, we highlight the importance of microbial research in general and the journal’s intention to serve as a publishing forum that supports and enfolds the scientific diversity in this area as it provides a unique, high-quality and universally accessible source of information and inspiration.
What’s the role of autophagy in trypanosomes?
Katherine Figarella1 and Néstor L. Uzcátegui1,2
This article comments on Proto et al. (Microbial Cell, 2014), who report first insights into the molecular mechanism of autophagy in African trypanosomes by generating reporter bloodstream form cell lines.
Microbial Cell
is an open-access, peer-reviewed journal that publishes exceptionally relevant research works that implement the use of unicellular organisms (and multicellular microorganisms) to understand cellular responses to internal and external stimuli and/or human diseases.
you can trust
Can’t find what you’re looking for?
You can browse all our issues and published articles here.
FAQs
Peer-reviewed, open-access research using unicellular organisms (and multicellular microorganisms) to understand cellular responses and human disease.
The journal (founded in 2014) is led by its Editors-in-Chief Frank Madeo, Didac Carmona-Gutierrez, and Guido Kroemer
Microbial Cell has been publishing original scientific literature since 2014, and from the very beginning has been managed by active scientists through an independent Publishing House (Shared science Publishers). The journal was conceived as a platform to acknowledge the importance of unicellular organisms, both as model systems as well as in the biological context of human health and disease.
Ever since, Microbial Cell has very positively developed and strongly grown into a respected journal in the unicellular research community and even beyond. This scientific impact is reflected in the yearly number of citations obtained by articles published in Microbial Cell, as recorded by the Web of Science (Clarivate, formerly Thomson/Reuters):
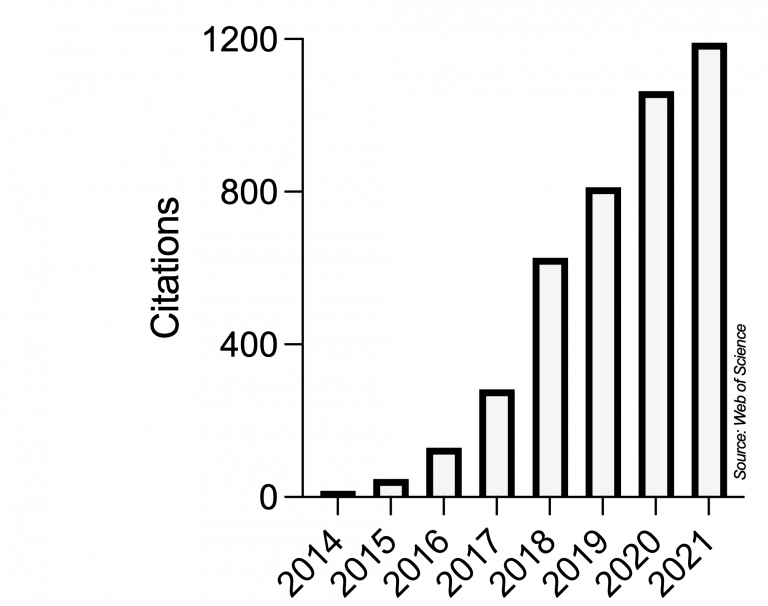
The scientific impact of Microbial Cell is also mirrored in a series of milestones:
2015: Microbial Cell is included in the Emerging Sources Citation Index (ESCI), a selection of developing journals drafted by Clarivate Analytics based on the candidate’s publishing standards, quality, editorial content, and citation data. Note: As an ESCI-selected journal, Microbial Cell is currently being evaluated in a rigorous and long process to determine an inclusion in the Science Citation Index Expanded (SCIE), which allows the official calculation of Clarivate Analytics’ impact factor.
2016: Microbial Cell is awarded the so-called DOAJ Seal by the selective Directory of Open Access Journals (DOAJ). The DOAJ Seal is an exclusive mark of certification for open access journals granted by DOAJ to journals that adhere to outstanding best practice and achieve an extra high and clear commitment to open access and high publishing standards.
2017: Microbial Cell is included in Pubmed Central (PMC), allowing the archiving of all the journal’s articles in PMC and PubMed.
2019: Microbial Cell is indexed in the prestigious abstract and citation database Scopus after a thorough selection process. This also means that Microbial Cell obtains, for the first time, an official Scopus CiteScore as well as an official journal ranking in the Scimago Journal and Country Ranking.
2022: Microbial Cell’s CiteScore reaches a value of 7.2 for the year 2021, positioning Microbial Cell among the top microbiology journals (previously available CiteScores: 2019: 5.4; 2020: 5.1).
2022: Microbial Cell is indexed in the highly selective Science Citation Index Expanded™, which covers approx. 9,500 of the world’s most impactful journals across 178 scientific disciplines. In their journal selection and curation process, Clarivate´s editors apply 24 ‘quality’ criteria and four ‘impact’ criteria to select the most influential journals in their respective fields. This selection is also a pre-requisite for inclusion in the JCR, which features the impact factor.
2022: Microbial Cell is listed in the Journal Citation Reports™ (JCR), and obtains its first official Journal Impact Factor™ (JIF) for the year 2021: 5.316.Check Article Types and Manuscript Preparation guidelines. Submit online via Scholastica.

Metabolic pathways further increase the complexity of cell size control in budding yeast
Jorrit M. Enserink
This article comments on work published by Soma et al. (Microbial Cell, 2014), which teased apart the effect of metabolism and growth rate on setting of critical cell size in Saccharomyces cerevisiae.