
Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.

Luminal acetylation of microtubules is not essential for Plasmodium berghei and Toxoplasma gondii survival
Acetylation of α-tubulin at lysine 40 is not essential for cytoskeletal stability in Plasmodium berghei or Toxoplasma gondii, suggesting redundancy and plasticity in microtubule regulation in these parasites.

The dual-site agonist for human M2 muscarinic receptors Iper-8-naphtalimide induces mitochondrial dysfunction in Saccharomyces cerevisiae
S. cerevisiae is a model to study human GPCRs. N-8-Iper, active against glioblastoma via M2 receptor, causes mitochondrial damage in yeast by binding Ste2, highlighting evolutionary conservation of GPCRs.

Integrative Omics reveals changes in the cellular landscape of peroxisome-deficient pex3 yeast cells
To uncover the consequences of peroxisome deficiency, we compared Saccharomyces cerevisiae wild-type with pex3 cells, which lack peroxisomes, employing quantitative proteomics and transcriptomics technologies.

Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
Rebekkah E. Pope1, Patrick Ballmann2, Lisa Whitworth3 and Rolf A. Prade1,*
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
Evelyn Tevere1,a, María G. Mediavilla1,a, Cecilia B. Di Capua1, Marcelo L. Merli1, Carlos Robello2,3, Luisa Berná2,4 and Julia A. Cricco
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.
Sir2 regulates selective autophagy in stationary-phase yeast cells
Ji-In Ryua, Juhye Junga, and Jeong-Yoon Kim
This study establishes Sir2 as a previously unrecognized regulator of selective autophagy during the stationary phase and highlight how cells dynamically control organelle degradation.
Global translational impacts of the loss of the tRNA modification t6A in yeast
Patrick C. Thiaville1,2,3,4, Rachel Legendre4, Diego Rojas-Benítez5, Agnès Baudin-Baillieu4, Isabelle Hatin4, Guilhem Chalancon6, Alvaro Glavic5, Olivier Namy4, Valérie de Crécy-Lagard1,3
The universal tRNA modification t6A is found at position 37 of nearly all tRNAs decoding ANN codons. Analysis of codon occupancy rates suggests that one of the major roles of t6A is to homogenize the process of elongation by slowing the elongation rate at codons decoded by high abundance tRNAs and I34:C3 pairs while increasing the elongation rate of rare tRNAs and G34:U3 pairs. This work reveals that the consequences of t6A absence are complex and multilayered and has set the stage to elucidate the molecular basis of the observed phenotypes.
Ergosterone-coupled Triazol molecules trigger mitochondrial dysfunction, oxidative stress, and acidocalcisomal Ca2+ release in Leishmania mexicana promastigotes
Figarella K1, Marsiccobetre S1, Arocha I1, Colina W2, Hasegawa M2,†, Rodriguez M2, Rodriguez-Acosta A3, Duszenko M4, Benaim G5, Uzcategui NL3
The protozoan parasite Leishmania causes a variety of sicknesses with different clinical manifestations known as leishmaniasis. Investigations looking for new targets or new active molecules focus mainly on the disruption of parasite specific pathways. In this sense, ergosterol biosynthesis is one of the most attractive because it does not occur in mammals. Our results indicate that ergosterone-triazol coupled molecules induce a regulated cell death process in the parasite and may represent starting point molecules in the search of new chemotherapeutic agents to combat leishmaniasis.
INO1 transcriptional memory leads to DNA zip code-dependent interchromosomal clustering
Donna Garvey Brickner, Robert Coukos and Jason H. Brickner
Many genes localize at the nuclear periphery through physical interaction with the nuclear pore complex (NPC). We have found that the yeast INO1 gene is targeted to the NPC both upon activation and for several generations after repression, a phenomenon called epigenetic transcriptional memory. Targeting of INO1 to the NPC requires distinct cis-acting promoter DNA zip codes under activating conditions and under memory conditions. When at the nuclear periphery, active INO1 clusters with itself and with other genes that share the GRS I zip code. Here, we show that during memory, the two alleles of INO1 cluster in diploids and endogenous INO1 clusters with an ectopic INO1 in haploids. After repression, INO1 does not cluster with GRS I – containing genes. Furthermore, clustering during memory requires Nup100 and two sets of DNA zip codes…
A central role for TOR signalling in a yeast model for juvenile CLN3 disease
Michael E. Bond1, Rachel Brown1, Charalampos Rallis3,4, Jürg Bähler3,4 and Sara E. Mole1,2,3
Yeasts provide an excellent genetically tractable eukaryotic system for investigating the function of genes in their biological context, and are especially relevant for those conserved genes that cause disease. Bond et al. study the role of btn1, the orthologue of a human gene that underlies an early onset neurodegenerative disease (juvenile CLN3 disease, neuronal ceroid lipofuscinosis (NCLs) or Batten disease) in the fission yeast Schizosaccharomyces pombe.
Oxygen availability strongly affects chronological lifespan and thermotolerance in batch cultures of Saccharomyces cerevisiae
Markus M.M. Bisschops1,3,#, Tim Vos1,#, Rubén Martínez-Moreno2,4, Pilar de la Torre Cortés1, Jack T. Pronk1, Pascale Daran-Lapujade1
Stationary-phase (SP) batch cultures of Saccharomyces cerevisiae, in which growth has been arrested by carbon-source depletion, are widely applied to study chronological lifespan, quiescence and SP-associated robustness. Based on this type of experiments, typically performed under aerobic conditions, several roles of oxygen in aging have been proposed. However, SP in anaerobic yeast cultures has not been investigated in detail. Here, we use the unique capability of S. cerevisiae to grow in the complete absence of oxygen to directly compare SP in aerobic and anaerobic bioreactor cultures. This comparison revealed strong positive effects of oxygen availability on adenylate energy charge, longevity and thermotolerance during SP. A low thermotolerance of…
DNA damage checkpoint adaptation genes are required for division of cells harbouring eroded telomeres
Sofiane Y. Mersaoui, Serge Gravel, Victor Karpov, and Raymund J. Wellinger
In budding yeast, telomerase and the Cdc13p protein are two key players acting to ensure telomere stability. This article shows that while the capping process can be flexible, it takes a very specific genetic setup to allow a change from canonical capping to alternative capping.
The MAPKKKs Ste11 and Bck1 jointly transduce the high oxidative stress signal through the cell wall integrity MAP kinase pathway
Chunyan Jin#, Stephen K. Kim, Stephen D. Willis and Katrina F. Cooper
Oxidative stress stimulates the Rho1 GTPase, which in turn induces the cell wall integrity (CWI) MAP kinase cascade. CWI activation promotes stress-responsive gene expression through activation of transcription factors (Rlm1, SBF) and nuclear release and subsequent destruction of the repressor cyclin C. This study reports that, in response to high hydrogen peroxide exposure, or in the presence of constitutively active Rho1, cyclin C still translocates to the cytoplasm and is degraded in cells lacking Bck1, the MAPKKK of the CWI pathway.
Formyl-methionine as a degradation signal at the N-termini of bacterial proteins
Konstantin I. Piatkov1,3,#, Tri T. M. Vu1,#, Cheol-Sang Hwang2 and Alexander Varshavsky1
Varshavsky and colleagues solve a long-standing mystery in proteolysis! In bacteria, all nascent proteins bear the pretranslationally formed N-terminal formyl-methionine (fMet) residue. The fMet residue is cotranslationally deformylated by a ribosome-associated deformylase. The formylation of N-terminal Met in bacterial proteins is not strictly essential for either translation or cell viability. Moreover, protein synthesis by the cytosolic ribosomes of eukaryotes does not involve the formylation of N-terminal Met. What, then, is the main biological function of this metabolically costly, transient, and not strictly essential modification of N‑terminal Met, and why has Met formylation not been eliminated during bacterial evolution? One possibility is that the similarity of the formyl and acetyl groups, their identical locations in…
Maintaining phagosome integrity during fungal infection: do or die?
Mabel Yang1, Glenn F.W. Walpole1,2 and Johannes Westman1
This article refers to the paper “Lysosome Fusion Maintains Phagosome Integrity during Fungal Infection” by Westman et al. (Cell Host Microbe, 2020), which shows that macrophages respond to pathogen growth by expanding the phagosome membrane through a calcium-dependent mechanism involving lysosome insertion, maintaining membrane integrity and preventing rupture.
Milestones in Bacillus subtilis sporulation research
Eammon P. Riley1, Corinna Schwarz2, Alan I. Derman2 and Javier Lopez-Garrido2
In this review, the foundational discoveries that shaped the sporulation field are discussed, from its origins to the present day, tracing a chronology that spans more than one hundred eighty years.
A novel antibacterial strategy: histone and antimicrobial peptide synergy
Leora Duong1, Steven P. Gross2,3 and Albert Siryaporn1,3
This article refers to the study “Mammalian histones facilitate antimicrobial synergy by disrupting the bacterial proton gradient and chromosome organization” by Doolin et al. (Nat Comm, 2020) that shows that histones enhance the antimicrobial activity of peptides, disrupt bacterial membranes, and inhibit transcription, offering new insights into natural antimicrobial mechanisms.
Extracellular vesicles: An emerging platform in gram-positive bacteria
Swagata Bose1,#, Shifu Aggarwal1,#, Durg Vijai Singh1,2 and Narottam Acharya1
Extracellular vesicles (EVs) are secreted by both pathogenic and non-pathogenic bacteria to transfer biomolecules and facilitate intercellular communication. While EV secretion in gram-negative bacteria is well understood, less is known about gram-positive bacteria. This review explores the role of EVs involved in bacterial competition, survival, immune evasion, and infection of gram-positive bacteria and compares them to gram-negative counterparts.
Structural insights into the architecture and assembly of eukaryotic flagella
Narcis-Adrian Petriman1 and Esben Lorentzen1
Cilia and flagella are key structures in motility and signaling. This review highlights recent findings of cryo-EM studies that have mapped the structure of axonemal microtubules in Chlamydomonas reinhardtii, revealing over 30 associated proteins as well as recent researcht which focused on the trafficking complexes that transport components between the cell body and cilium.
Erythrocyte phospho-signalling is dynamically altered during infection with Plasmodium falciparum
Jack D. Adderley1 and Christian Doerig1
This article refers to the study “Analysis of erythrocyte signalling pathways during Plasmodium falciparum infection identifies targets for host-directed antimalarial intervention” by Adderley et al. (Nat Commun, 2020) that investigates how Plasmodium falciparum malaria parasites influence red blood cells. By tracking hanges in over 800 human proteins at different parasite stages they confirmed activation of the PAK-MEK pathway and discovered significant changes, particularly during the trophozoite stage. This suggests that kinases activated by the infection could be targeted for new antimalarial therapies.
Plant and fungal products that extend lifespan in Caenorhabditis elegans
Jan Martel1,2, Cheng-Yeu Wu1-3, Hsin-Hsin Peng1,2,4, Yun-Fei Ko2,5,6, Hung-Chi Yang7, John D. Young5 and David M. Ojcius1,2,8
Caenorhabditis elegans’ lifespan is extended by plant and fungal extracts activating pathways like autophagy and mitochondrial biogenesis. Low to moderate concentrations promote longevity, while high doses are harmful. This review explores the health benefits of these substances in humans.
A new role for proteins subunits of RNase P: stabilization of the telomerase holoenzyme
P. Daniela Garcia1 and Virginia A. Zakian2
This article refers to the study “Stability and Nuclear Localization of Yeast Telomerase Depend on Protein Components of RNase P/MRP”, by Garcia et al. (Nat Commun, 2020), showing that 3 essential proteins in Saccharomyces cerevisiae are vital for telomerase assembly and nuclear localization. In their mutants, telomerase is less mature, and telomeres are shorter. TLC1 is properly folded but remains in the cytoplasm, rather than moving to the nucleus, where it maintains telomeres.
Lipid droplet biogenesis from specialized ER subdomains
Vineet Choudhary1 and Roger Schneiter2
This article refers to the paper “Seipin and Nem1 establish discrete ER subdomains to initiate yeast lipid droplet biogenesis” by Choudhary et al. (J Cell Biol, 2020), which deals with the formation of lipid droplets (LDs) at specific ER sites marked by the proteins Fld1 and Nem1. These proteins recruit enzymes such as Lro1 and Dga1 to initiate fat storage. Together, Fld1 and Nem1 define where LDs form by organising key proteins and lipids needed for their biogenesis.
Non-genetic impact factors on chronological lifespan and stress resistance of baker’s yeast
Michael Sauer and Diethard Mattanovich
This article comments on work published by Bisschops et al. (Microbial Cell, 2015), which illustrates how important the choice of the experimental setup is and how culture conditions influcence cellular aging and survival in biotechnological processes.
What’s old is new again: yeast mutant screens in the era of pooled segregant analysis by genome sequencing
Chris Curtin and Toni Cordente
This article comments on work published by Den Abt et al. (Microbial Cell, 2016), which identified genes involved in ethyl acetate formation in a yeast mutant screen based on a new approach combining repeated rounds of chemical mutagenesis and pooled segregant analysis by whole genome sequencing.
The complexities of bacterial-fungal interactions in the mammalian gastrointestinal tract
Eduardo Lopez-Medina1 and Andrew Y. Koh2
This article comments on work published by Lopez-Medina et al. (PLoS Pathog, 2015) and Fan et al. (Nat Med, 2015), which utilize an “artificial” niche, the antibiotic-treated gut with concomitant pathogenic microbe expansion, to gain insight in bacterial-fungal interactions in clinically common scenarios.
Gearing up for survival – HSP-containing granules accumulate in quiescent cells and promote survival
Ruofan Yu and Weiwei Dang
This article comments on work published by Lee et al. (Microbial Cell, 2016), which reports that distinct granules are formed in quiescent and non-quiescent cells, which determines their respective cell fates.
Yeast screening platform identifies FDA-approved drugs that reduce Aβ oligomerization
Triana Amen1,2 and Daniel Kaganovich1
This article comments on work published by Park et al. (Microbial Cell, 2016), which discovered a number of small molecules capable of modulating Aβ aggregation in a yeast model.
Groupthink: chromosomal clustering during transcriptional memory
Kevin A. Morano
In this article, the authors comment on the study “NO1 transcriptional memory leads to DNA zip code-dependent interchromosomal clustering.” by Brickner et al. (Microbial Cell, 2015), discussing the importance and molecular mechanisms of chromosomal clustering during transcriptional memory.
Yeast proteinopathy models: a robust tool for deciphering the basis of neurodegeneration
Amit Shrestha1, 2 and Lynn A. Megeney1, 2, 3
Protein quality control or proteostasis is an essential determinant of basic cell health and aging. Eukaryotic cells have evolved a number of proteostatic mechanisms to ensure that proteins retain functional conformation, or are rapidly degraded when proteins misfold or self-aggregate. This article discusses the use of budding yeast as a robust proxy to study the intersection between proteostasis and neurodegenerative disease.
Microbial Cell
is an open-access, peer-reviewed journal that publishes exceptionally relevant research works that implement the use of unicellular organisms (and multicellular microorganisms) to understand cellular responses to internal and external stimuli and/or human diseases.
you can trust
Can’t find what you’re looking for?
You can browse all our issues and published articles here.
FAQs
Peer-reviewed, open-access research using unicellular organisms (and multicellular microorganisms) to understand cellular responses and human disease.
The journal (founded in 2014) is led by its Editors-in-Chief Frank Madeo, Didac Carmona-Gutierrez, and Guido Kroemer
Microbial Cell has been publishing original scientific literature since 2014, and from the very beginning has been managed by active scientists through an independent Publishing House (Shared science Publishers). The journal was conceived as a platform to acknowledge the importance of unicellular organisms, both as model systems as well as in the biological context of human health and disease.
Ever since, Microbial Cell has very positively developed and strongly grown into a respected journal in the unicellular research community and even beyond. This scientific impact is reflected in the yearly number of citations obtained by articles published in Microbial Cell, as recorded by the Web of Science (Clarivate, formerly Thomson/Reuters):
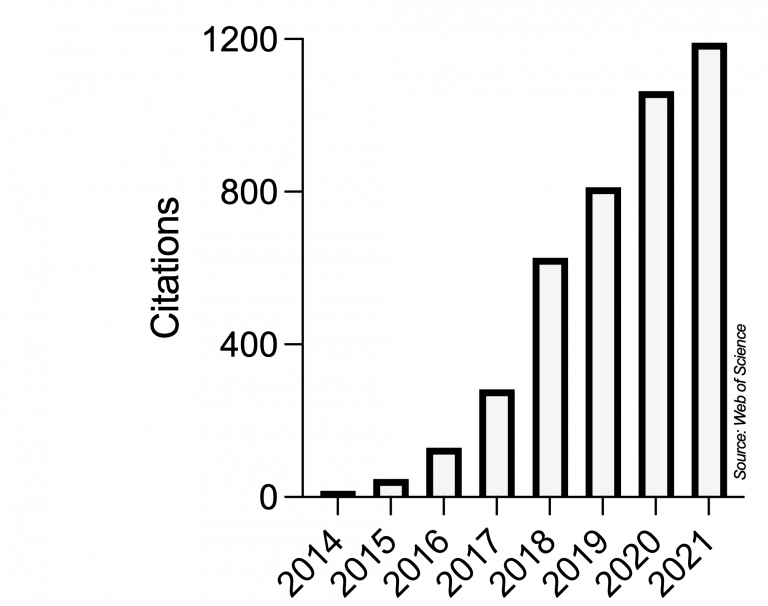
The scientific impact of Microbial Cell is also mirrored in a series of milestones:
2015: Microbial Cell is included in the Emerging Sources Citation Index (ESCI), a selection of developing journals drafted by Clarivate Analytics based on the candidate’s publishing standards, quality, editorial content, and citation data. Note: As an ESCI-selected journal, Microbial Cell is currently being evaluated in a rigorous and long process to determine an inclusion in the Science Citation Index Expanded (SCIE), which allows the official calculation of Clarivate Analytics’ impact factor.
2016: Microbial Cell is awarded the so-called DOAJ Seal by the selective Directory of Open Access Journals (DOAJ). The DOAJ Seal is an exclusive mark of certification for open access journals granted by DOAJ to journals that adhere to outstanding best practice and achieve an extra high and clear commitment to open access and high publishing standards.
2017: Microbial Cell is included in Pubmed Central (PMC), allowing the archiving of all the journal’s articles in PMC and PubMed.
2019: Microbial Cell is indexed in the prestigious abstract and citation database Scopus after a thorough selection process. This also means that Microbial Cell obtains, for the first time, an official Scopus CiteScore as well as an official journal ranking in the Scimago Journal and Country Ranking.
2022: Microbial Cell’s CiteScore reaches a value of 7.2 for the year 2021, positioning Microbial Cell among the top microbiology journals (previously available CiteScores: 2019: 5.4; 2020: 5.1).
2022: Microbial Cell is indexed in the highly selective Science Citation Index Expanded™, which covers approx. 9,500 of the world’s most impactful journals across 178 scientific disciplines. In their journal selection and curation process, Clarivate´s editors apply 24 ‘quality’ criteria and four ‘impact’ criteria to select the most influential journals in their respective fields. This selection is also a pre-requisite for inclusion in the JCR, which features the impact factor.
2022: Microbial Cell is listed in the Journal Citation Reports™ (JCR), and obtains its first official Journal Impact Factor™ (JIF) for the year 2021: 5.316.Check Article Types and Manuscript Preparation guidelines. Submit online via Scholastica.

Similar environments but diverse fates: Responses of budding yeast to nutrient deprivation.
Saul M. Honigberg
Diploid budding yeast (Saccharomyces cerevisiae) can adopt one of several alternative differentiation fates in response to nutrient limitation, and each of these fates provides distinct biological functions. When different strain backgrounds are taken into account, these various fates occur in response to similar environmental cues, are regulated by the same signal transduction pathways, and share many of the same master regulators. I propose that the relationships between fate choice, environmental cues and signaling pathways are not Boolean, but involve graded levels of signals, pathway activation and master-regulator activity.