
Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.

Luminal acetylation of microtubules is not essential for Plasmodium berghei and Toxoplasma gondii survival
Acetylation of α-tubulin at lysine 40 is not essential for cytoskeletal stability in Plasmodium berghei or Toxoplasma gondii, suggesting redundancy and plasticity in microtubule regulation in these parasites.

The dual-site agonist for human M2 muscarinic receptors Iper-8-naphtalimide induces mitochondrial dysfunction in Saccharomyces cerevisiae
S. cerevisiae is a model to study human GPCRs. N-8-Iper, active against glioblastoma via M2 receptor, causes mitochondrial damage in yeast by binding Ste2, highlighting evolutionary conservation of GPCRs.

Integrative Omics reveals changes in the cellular landscape of peroxisome-deficient pex3 yeast cells
To uncover the consequences of peroxisome deficiency, we compared Saccharomyces cerevisiae wild-type with pex3 cells, which lack peroxisomes, employing quantitative proteomics and transcriptomics technologies.

Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
Rebekkah E. Pope1, Patrick Ballmann2, Lisa Whitworth3 and Rolf A. Prade1,*
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
Evelyn Tevere1,a, María G. Mediavilla1,a, Cecilia B. Di Capua1, Marcelo L. Merli1, Carlos Robello2,3, Luisa Berná2,4 and Julia A. Cricco
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.
Sir2 regulates selective autophagy in stationary-phase yeast cells
Ji-In Ryua, Juhye Junga, and Jeong-Yoon Kim
This study establishes Sir2 as a previously unrecognized regulator of selective autophagy during the stationary phase and highlight how cells dynamically control organelle degradation.
Persistence phenotype of adherent-invasive Escherichia coli in response to ciprofloxacin, revealing high-persistence strains
Valeria Pérez-Villalobos1, Roberto Vidal2, Marcela A. Hermoso3,4 and Paula Bustamante1
We investigated the roles of the resident antibiotic resistance plasmid, the stress response protein HtrA, and macrophage-induced persister formation. Our results revealed broad variability in persister cell formation among AIEC strains.
Knocking out histidine ammonia-lyase by using CRISPR-Cas9 abolishes histidine role in the bioenergetics and the life cycle of Trypanosoma cruzi
Janaína de Freitas Nascimento1, María Julia Barisón1, Gabriela Torres Montanaro1, Letícia Marchese1, Rodolpho Ornitz Oliveira Souza1, Letícia Sophia Silva2, Alessandra Aparecida Guarnieri2 and Ariel Mariano Silber1
Recent studies have highlighted the importance of this pathway in ATP production, redox balance, and the maintenance of cellular homeostasis in T. cruzi. In this work, we focus on the first step of the histidine degradation pathway, which is performed by the enzyme histidine ammonia lyase. Here we determined the kinetic and biochemical parameters of the T. cruzi histidine ammonia-lyase.
Dissecting the cell cycle regulation, DNA damage sensitivity and lifespan effects of caffeine in fission yeast
John-Patrick Alao1, Juhi Kumar1, Despina Stamataki2 and Charalampos Rallis1
Our findings show that caffeine accelerates mitotic division and is beneficial for CLS through AMPK. Direct pharmacological targeting of AMPK may serve towards healthspan and lifespan benefits beyond yeasts, given the highly conserved nature of this key regulatory cellular energy sensor.

Ampicillin treatment in persister cell studies may cause non-physiological artifacts
Michel Fasnacht1,2, Hena Comic1,2, Isabella Moll1,2
This study shows at the example of L2 how insufficient purification of ampicillin persister cells can lead to the generation of non-physiological artifacts and provides a novel tool to improve the removal of residual cell debris.

Clostridium scindens promotes gallstone formation by inducing intrahepatic neutrophil extracellular traps through CXCL1 produced by colonic epithelial cells
Wenchao Yao1,a, Yuanhang He2,3,a, Zhihong Xie2,3, Qiang Wang2,3, Yang Chen2,4, Jingjing Yu2,3, Xuxu Liu2,3, Dongbo Xue2,3 , Liyi Wang2,3 and Chenjun Hao2,3
Through in vivo and in vitro experiments, we validated the reliability of C. scindens stimulating colonic epithelial cells to produce TLR2, activating the NF-κB signaling pathway, promoting CXCL1 expres-sion, and inducing intrahepatic neutrophil NETosis, which may be associated with gallstone formation.

Integrative Omics reveals changes in the cellular landscape of peroxisome-deficient pex3 yeast cells
Tjasa Kosir1,a, Hirak Das2,a, Marc Pilegaard Pedersen1, Ann-Kathrin Richard2, Marco Anteghini3,4, Vitor Martins dos Santos4,5, Silke Oeljeklaus2, Ida J. van der Klei1 and Bettina Warscheid2
To uncover the consequences of peroxisome deficiency, we compared Saccharomyces cerevisiae wild-type with pex3 cells, which lack peroxisomes, employing quantitative proteomics and transcriptomics technologies.
A roadmap for designing narrow-spectrum antibiotics targeting bacterial pathogens
Xinyun Cao1,*, Robert Landick1,2, Elizabeth A. Campbell3
This comment discusses the article “Basis of narrow-spectrum activity of fidaxomicin on Clostridioides difficile” by Cao et al. (2022, Nature).
Breaking the clip for cargo unloading from motor proteins: mechanism and significance
Keisuke Obara1, and Takumi Kamura1
The mitochondrion is an essential organelle involved in ATP generation, lipid metabolism, regulation of calcium ions, etc. Therefore, it should be inherited properly by newly generated cells. In the budding yeast Saccharomyces cerevisiae, mitochondria are passed on to daughter cells by the motor protein, Myo2, on the actin cable. The mitochondria and Myo2 are connected via the adaptor protein Mmr1. After reaching daughter cells, mitochondria are released from the actin-myosin machinery and move dynamically. In our recent paper (Obara K et al. (2022), Nat Commun, doi:10.1038/s41467-022-29704-8), we demonstrated that the regulated proteolysis of Mmr1 is required for the unloading of mitochondria from Myo2 in daughter cells. Sequential post-translational modifications of Mmr1, i.e., phosphorylation followed by ubiquitination, are essential for Mmr1 degradation and mitochondrial release from Myo2. Defects in Mmr1 degradation cause stacking and deformation of mitochondria at the bud-tip and bud-neck, where Myo2 accumulates. Compared to wild-type cells, mutant cells with defects in Mmr1 degradation possess an elevated mitochondrial membrane potential and produce higher levels of reactive oxygen species (ROS), along with hypersensitivity to oxidative stress.
Pirates of the haemoglobin
Daniel Akinbosede1, Robert Chizea1 and Stephen A. Hare1,†
Not all treasure is silver and gold; for pathogenic bacteria, iron is the most precious and the most pillaged of metallic elements. Iron is essential for the survival and growth of all life; however free iron is scarce for bacteria inside human hosts. As a mechanism of defence, humans have evolved ways to store iron so as to render it inaccessible for invading pathogens, such as keeping the metal bound to iron-carrying proteins. For bacteria to survive within humans, they must therefore evolve counters to this defence to compete with these proteins for iron binding, or directly steal iron from them. (…)
An ionophore breaks the multi-drug-resistance of Acinetobacter baumannii
David M.P. De Oliveira1 and Mark J. Walker1
Within intensive care units, multi-drug resistant Acinetobacter baumannii outbreaks are a frequent cause of ventilator-associated pneumonia. During the on-going COVID-19 pandemic, patients who receive ventilator support experience a 2-fold increased risk of mortality when they contract a secondary A. baumannii pulmonary infection. In our recent paper (De Oliveira et al. (2022), Mbio, doi: 10.1128/mbio.03517-21), we demonstrate that the 8-hydroxquinoline ionophore, PBT2 breaks the resistance of A. baumannii to tetracycline class antibiotics. In vitro, the combination of PBT2 and zinc with either tetracycline, doxycycline, or tigecycline was shown to be bactericidal against multi-drug-resistant A. baumannii, (…)
Endomembrane remodeling and dynamics in Salmonella infection
Ziyan Fang1 and Stéphane Méresse1
Salmonellae are bacteria that cause moderate to severe infections in humans, depending on the strain and the immune status of the infected host. These pathogens have the particularity of residing in the cells of the infected host. They are usually found in a vacuolar compartment that the bacteria shape with the help of effector proteins. Following invasion of a eukaryotic cell, the bacterial vacuole undergoes maturation characterized by changes in localization, composition and morphology. In particular, membrane tubules stretching over the microtubule cytoskeleton are formed from the bacterial vacuole. Although these tubules do not occur in all infected cells, they are functionally important and promote intracellular replication. This review focuses on the role and significance of membrane compartment remodeling observed in infected cells and the bacterial and host cell pathways involved.
The small bowel microbiome changes significantly with age and aspects of the ageing process
Gabriela Leite1, Mark Pimentel1,2, Gillian M. Barlow1 and Ruchi Mathur1,3
Gut microbiome changes have been associated with human ageing and implicated in age-related diseases including Alzheimer’s disease and Parkinson’s disease. However, studies to date have used stool samples, which do not represent the entire gut. Although more challenging to access, the small intestine plays critical roles in host metabolism and immune function. In this paper (Leite et al. (2021), Cell Reports, doi: 10.1016/j.celrep.2021.109765), we demonstrate significant differences in the small intestinal microbiome in older subjects, (…)
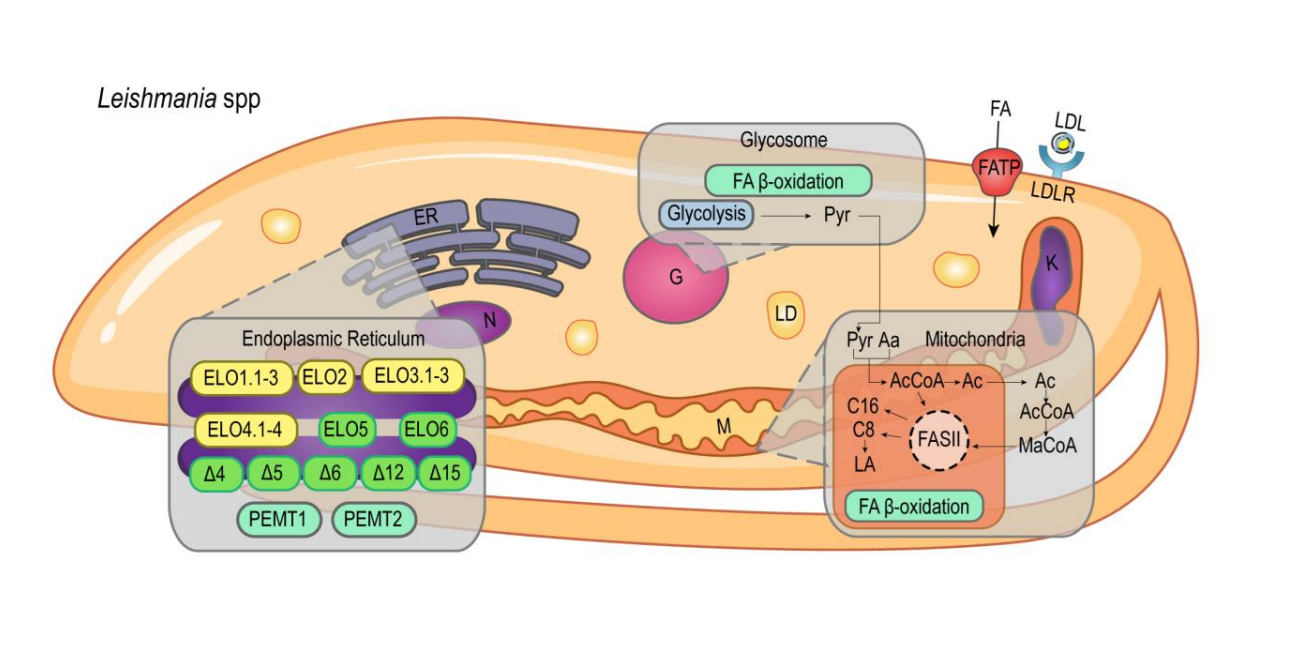
Lipid and fatty acid metabolism in trypanosomatids
Giovana Parreira de Aquino1,#, Marco Antonio Mendes Gomes1,#, Roberto Köpke Salinas2 and Maria Fernanda Laranjeira-Silva1
This work reviews specific aspects of lipid and fatty acid metabolism in the protozoan parasites T. brucei, T. cruzi, and Leishmania spp., as well as the pathways that have been explored for the development of new chemotherapies.
The emerging role of complex modifications of tRNALysUUU in signaling pathways
Patrick C. Thiaville1,2,3,4 and Valérie de Crécy-Lagard2,4
This comment discusses the article “Loss of wobble uridine modification in tRNA anticodons interferes with TOR pathway signaling” by Scheidt et al (Microbial Cell, 2014).
Only functional localization is faithful localization
Roland Lill1,2,3
This article comments on work published by Peleh et al. (Microbial Cell 2014), which analyzes the localization of Dre2 in Saccharomyces cerevisiae.
One cell, one love: a journal for microbial research
Didac Carmona-Gutierrez1, Guido Kroemer2-6 and Frank Madeo1
In this inaugural article of Microbial Cell, we highlight the importance of microbial research in general and the journal’s intention to serve as a publishing forum that supports and enfolds the scientific diversity in this area as it provides a unique, high-quality and universally accessible source of information and inspiration.
What’s the role of autophagy in trypanosomes?
Katherine Figarella1 and Néstor L. Uzcátegui1,2
This article comments on Proto et al. (Microbial Cell, 2014), who report first insights into the molecular mechanism of autophagy in African trypanosomes by generating reporter bloodstream form cell lines.
Microbial Cell
is an open-access, peer-reviewed journal that publishes exceptionally relevant research works that implement the use of unicellular organisms (and multicellular microorganisms) to understand cellular responses to internal and external stimuli and/or human diseases.
you can trust
Can’t find what you’re looking for?
You can browse all our issues and published articles here.
FAQs
Peer-reviewed, open-access research using unicellular organisms (and multicellular microorganisms) to understand cellular responses and human disease.
The journal (founded in 2014) is led by its Editors-in-Chief Frank Madeo, Didac Carmona-Gutierrez, and Guido Kroemer
Microbial Cell has been publishing original scientific literature since 2014, and from the very beginning has been managed by active scientists through an independent Publishing House (Shared science Publishers). The journal was conceived as a platform to acknowledge the importance of unicellular organisms, both as model systems as well as in the biological context of human health and disease.
Ever since, Microbial Cell has very positively developed and strongly grown into a respected journal in the unicellular research community and even beyond. This scientific impact is reflected in the yearly number of citations obtained by articles published in Microbial Cell, as recorded by the Web of Science (Clarivate, formerly Thomson/Reuters):
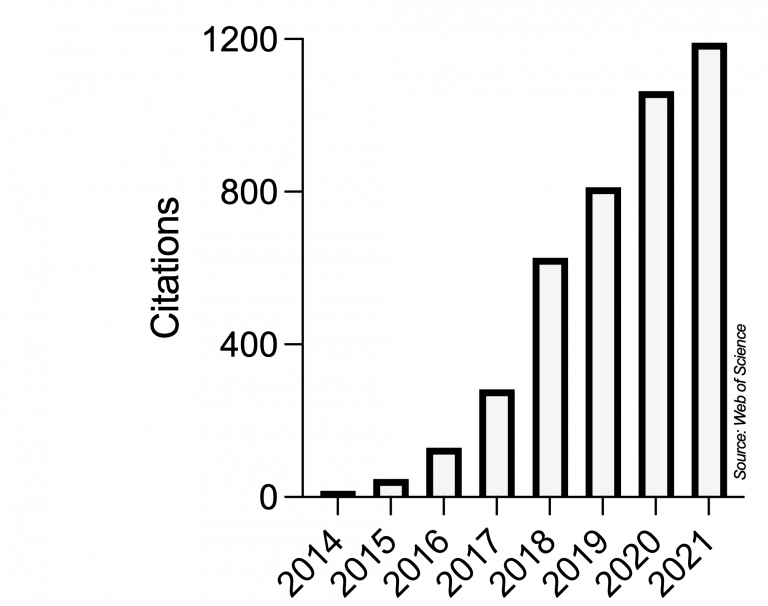
The scientific impact of Microbial Cell is also mirrored in a series of milestones:
2015: Microbial Cell is included in the Emerging Sources Citation Index (ESCI), a selection of developing journals drafted by Clarivate Analytics based on the candidate’s publishing standards, quality, editorial content, and citation data. Note: As an ESCI-selected journal, Microbial Cell is currently being evaluated in a rigorous and long process to determine an inclusion in the Science Citation Index Expanded (SCIE), which allows the official calculation of Clarivate Analytics’ impact factor.
2016: Microbial Cell is awarded the so-called DOAJ Seal by the selective Directory of Open Access Journals (DOAJ). The DOAJ Seal is an exclusive mark of certification for open access journals granted by DOAJ to journals that adhere to outstanding best practice and achieve an extra high and clear commitment to open access and high publishing standards.
2017: Microbial Cell is included in Pubmed Central (PMC), allowing the archiving of all the journal’s articles in PMC and PubMed.
2019: Microbial Cell is indexed in the prestigious abstract and citation database Scopus after a thorough selection process. This also means that Microbial Cell obtains, for the first time, an official Scopus CiteScore as well as an official journal ranking in the Scimago Journal and Country Ranking.
2022: Microbial Cell’s CiteScore reaches a value of 7.2 for the year 2021, positioning Microbial Cell among the top microbiology journals (previously available CiteScores: 2019: 5.4; 2020: 5.1).
2022: Microbial Cell is indexed in the highly selective Science Citation Index Expanded™, which covers approx. 9,500 of the world’s most impactful journals across 178 scientific disciplines. In their journal selection and curation process, Clarivate´s editors apply 24 ‘quality’ criteria and four ‘impact’ criteria to select the most influential journals in their respective fields. This selection is also a pre-requisite for inclusion in the JCR, which features the impact factor.
2022: Microbial Cell is listed in the Journal Citation Reports™ (JCR), and obtains its first official Journal Impact Factor™ (JIF) for the year 2021: 5.316.Check Article Types and Manuscript Preparation guidelines. Submit online via Scholastica.



Metabolic pathways further increase the complexity of cell size control in budding yeast
Jorrit M. Enserink
This article comments on work published by Soma et al. (Microbial Cell, 2014), which teased apart the effect of metabolism and growth rate on setting of critical cell size in Saccharomyces cerevisiae.