
Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.

Luminal acetylation of microtubules is not essential for Plasmodium berghei and Toxoplasma gondii survival
Acetylation of α-tubulin at lysine 40 is not essential for cytoskeletal stability in Plasmodium berghei or Toxoplasma gondii, suggesting redundancy and plasticity in microtubule regulation in these parasites.

The dual-site agonist for human M2 muscarinic receptors Iper-8-naphtalimide induces mitochondrial dysfunction in Saccharomyces cerevisiae
S. cerevisiae is a model to study human GPCRs. N-8-Iper, active against glioblastoma via M2 receptor, causes mitochondrial damage in yeast by binding Ste2, highlighting evolutionary conservation of GPCRs.

Integrative Omics reveals changes in the cellular landscape of peroxisome-deficient pex3 yeast cells
To uncover the consequences of peroxisome deficiency, we compared Saccharomyces cerevisiae wild-type with pex3 cells, which lack peroxisomes, employing quantitative proteomics and transcriptomics technologies.

Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
Rebekkah E. Pope1, Patrick Ballmann2, Lisa Whitworth3 and Rolf A. Prade1,*
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
Evelyn Tevere1,a, María G. Mediavilla1,a, Cecilia B. Di Capua1, Marcelo L. Merli1, Carlos Robello2,3, Luisa Berná2,4 and Julia A. Cricco
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.
Sir2 regulates selective autophagy in stationary-phase yeast cells
Ji-In Ryua, Juhye Junga, and Jeong-Yoon Kim
This study establishes Sir2 as a previously unrecognized regulator of selective autophagy during the stationary phase and highlight how cells dynamically control organelle degradation.
A single mutation in the 15S rRNA gene confers non sense suppressor activity and interacts with mRF1 the release factor in yeast mitochondria
Ali Gargouri, Catherine Macadré and Jaga Lazowska
This article presents the nucleotide sequence of the mim3-1 mitochondrial ribosomal suppressor, acting on ochre mitochondrial mutations and one frameshift mutation in Saccharomyces cerevisiae. A hypothetical mechanism of suppression by “ribosome shifting” is also discussed in view of the nature of mutations suppressed and not suppressed.
The lysosomotropic drug LeuLeu-OMe induces lysosome disruption and autophagy-independent cell death in Trypanosoma brucei
Hazel Xinyu Koh1,2, Htay Mon Aye1, Kevin S. W. Tan2 and Cynthia Y. He1
Trypanosoma brucei is a blood-borne, protozoan parasite that causes African sleeping sickness in humans and nagana in animals. The current chemotherapy relies on only a handful of drugs that display undesirable toxicity, poor efficacy and drug-resistance. In this study, we explored the use of lysosomotropic drugs to induce bloodstream form T. brucei cell death via lysosome destabilization. We measured drug concentrations that inhibit cell proliferation by 50% (IC50) for several compounds, chosen based on their lysosomotropic effects previously reported in Plasmodium falciparum. The lysosomal effects and cell death induced by L-leucyl-L-leucyl methyl ester (LeuLeu-OMe) were further analyzed by flow cytometry and immunofluorescence analyses of different lysosomal markers…
In Entamoeba histolytica, a BspA family protein is required for chemotaxis toward tumour necrosis factor
Anne Silvestre1, 2, 3, 4, Aurélie Plaze1, 2, Patricia Berthon3, 4, Roman Thibeaux1, 2, Nancy Guillen1, 2 and Elisabeth Labruyère1, 2
Background: Entamoeba histolytica cell migration is essential for the development of human amoebiasis (an infectious disease characterized by tissue invasion and destruction). The tissue inflammation associated with tumour necrosis factor (TNF) secretion by host cells is a well-documented feature of amoebiasis. Tumour necrosis factor is a chemoattractant for E. histolytica, and the parasite may have a TNF receptor at its cell surface. Methods: confocal microscopy, RNA Sequencing, bioinformatics, RNA antisense techniques and histological analysis of human colon explants were used to characterize the interplay between TNF and E. histolytica. Results: an antibody against human TNF receptor 1 (TNFR1) stained the E. histolytica trophozoite…
Human Thyroid Cancer-1 (TC-1) is a vertebrate specific oncogenic protein that protects against copper and pro-apoptotic genes in yeast
Natalie K. Jones1,2,4,#, Nagla T.T. Arab1,3,#, Rawan Eid1,3,#, Nada Gharib1,5, Sara Sheibani1,2,6, Hojatollah Vali2, Chamel Khoury1, Alistair Murray1,2, Eric Boucher2, Craig A. Mandato2, Paul G. Young3 and Michael T. Greenwood1
The human Thyroid Cancer-1 (hTC-1) protein, also known as C8orf4 was initially identified as a gene that was up-regulated in human thyroid cancer. This article reports that hTC-1 is a peptide that prevents the effects of over-expressing Bax in yeast. In sum, the results indicate that hTC-1 is a pro-survival protein that retains its function when heterologously expressed in yeast. Thus yeast is a useful model to characterize the potential roles in cell death and survival of cancer related genes.
Polyamines directly promote antizyme-mediated degradation of ornithine decarboxylase by the proteasome
R. Roshini Beenukumar1,#, Daniela Gödderz1,2,#, R. Palanimurugan1,3, and R. Jürgen Dohmen1
Ornithine decarboxylase (ODC), a ubiquitin-independent substrate of the proteasome, is a homodimeric protein with a rate-limiting function in polyamine biosynthesis. Polyamines regulate ODC levels by a feedback mechanism mediated by ODC antizyme (OAZ). Higher cellular polyamine levels trigger the synthesis of OAZ and also inhibit its ubiquitin-dependent proteasomal degradation. OAZ binds ODC monomers and targets them to the proteasome. Here, we report that polyamines, aside from their role in the control of OAZ synthesis and stability, directly enhance OAZ-mediated ODC degradation by the proteasome. Using a stable mutant of OAZ, we show that polyamines promote ODC degradation in Saccharomyces cerevisiae cells even when OAZ levels are not changed. Furthermore, polyamines stimulated the in vitro degradation of ODC by the…
Toxoplasma gondii inhibits cytochrome c-induced caspase activation in its host cell by interference with holo-apoptosome assembly
Kristin Graumann1,3,#, Frieder Schaumburg1,4,#, Thomas F. Reubold2, Diana Hippe1, Susanne Eschenburg2 and Carsten G. K. Lüder1
Inhibition of programmed cell death pathways of mammalian cells often facilitates the sustained survival of intracellular microorganisms. The apicomplexan parasite Toxoplasma gondii is a master regulator of host cell apoptotic pathways. Here, we have characterized a novel anti-apoptotic activity of T. gondii. Using a cell-free cytosolic extract model, we show that T. gondii interferes with the activities of caspase 9 and caspase 3/7 which have been induced by exogenous cytochrome c and dATP. Proteolytic cleavage of caspases 9 and 3 is also diminished suggesting inhibition of holo-apoptosome function. Parasite infection of Jurkat T cells and subsequent triggering of apoptosome formation by exogenous cytochrome c in vitro and in vivo indicated that…
Exogenous folates stimulate growth and budding of Candida glabrata
Afsaneh Porzoor and Ian G. Macreadie
Folate, vitamin B9, is well recognized as being essential for cell growth. The utilization of folate is common to all cells, but the source of it may be quite different. This article reports a novel response of yeast to folates that may increase the utility of yeast as a model to study folate transport and signaling.
Modeling human Coenzyme A synthase mutation in yeast reveals altered mitochondrial function, lipid content and iron metabolism
Camilla Ceccatelli Berti1, Cristina Dallabona1, Mirca Lazzaretti1, Sabrina Dusi2, Elena Tosi1, Valeria Tiranti2, Paola Goffrini1
Mutations in nuclear genes associated with defective coenzyme A biosynthesis have been identified as responsible for some forms of neurodegeneration with brain iron accumulation (NBIA), namely PKAN and CoPAN. Yeast expressing a pathogenic mutation exhibited a temperature-sensitive growth defect in the absence of pantothenate and a reduced CoA content. Additional characterization revealed decreased oxygen consumption, reduced activities of mitochondrial respiratory complexes, higher iron content, increased sensitivity to oxidative stress and reduced amount of lipid droplets, thus partially recapitulating the phenotypes found in patients and establishing yeast as a potential model to clarify the pathogenesis underlying PKAN and CoPAN diseases.
Metabolic disharmony and sibling conflict mediated by T6SS
Vera Troselj1 and Daniel Wall1
In this article, the authors comment on the study “Physiological Heterogeneity Triggers Sibling Conflict Mediated by the Type VI Secretion System in an Aggregative Multicellular Bacterium” by Troselj et al. (MBio, 2018) discussing that M. xanthus uses T6SS to eliminate less fit cells from their population and identified toxic effector and cognate immunity protein (TsxEI) that mediates this sibling antagonism.
Helicobacter hepaticus polysaccharide induces an anti-inflammatory response in intestinal macrophages
Camille Danne1 and Fiona Powrie1
In this article, the authors comment on the study “A Large Polysaccharide Produced by Helicobacter hepaticus Induces an Anti-inflammatory Gene Signature in Macrophages. ” by Danne et al, (Cell Host Microbe 2017), discussing the interactions between H. hepaticus and intestinal macrophages that promote mutualism.
Endolysosomal pathway activity protects cells from neurotoxic TDP-43
Christine Leibiger1,#, Jana Deisel1,#, Andreas Aufschnaiter2, Stefanie Ambros1, Maria Tereshchenko1, Bert M. Verheijen3,4, Sabrina Büttner2,5, and Ralf J. Braun1
In this article, the authors comment on the study “TDP-43 controls lysosomal pathways thereby determining its own clearance and cytotoxicity” by Leibiger et al. (Hum Mol Genet, 2018), proposing that ameliorating endolysosomal pathway activity enhances cell survival in TDP‑43-associated diseases.
Two distinct penicillin binding proteins promote cell division in different Salmonella lifestyles
Sónia Castanheira1, Juan J. Cestero1, Francisco García-del Portillo1, M. Graciela Pucciarelli1,2,3
In this article, the authors comment on the study “A Specialized Peptidoglycan Synthase Promotes Salmonella Cell Division inside Host Cells” by Castanheira et al. (mBio, 2017), discussing insights in two distinct penicillin binding proteins that promote cell division in different Salmonella lifestyles.
New perspectives from South-Y-East, not all about deathA report of the 12th lnternational Meeting on Yeast Apoptosis in Bari, Italy, May 14th-18th, 2017
Nicoletta Guaragnella1,#, Mariarita Stirpe2,#, William Burhans3, Manuela Côrte-Real4, Campbell Gourlay5, Paula Ludovico6,7, Frank Madeo8,9, Dina Petranovic10, Joris Winderickx11, Cristina Mazzoni2 and Sergio Giannattasio1
In this article Guaragnella et al. report on the 12th International Meeting on Yeast Apoptosis (IMYA12), which was held in Bari, Italy from May 14th to 18th, 2017, where more than 100 participants, among which senior and young scientists from Europe, USA, North Africa and Japan, had an intense and open exchange of achievements and ideas in the field of yeast regulated cell death (RCD).
pH homeostasis links the nutrient sensing PKA/TORC1/Sch9 ménage-à-trois to stress tolerance and longevity
Marie-Anne Deprez1,°, Elja Eskes1,°, Tobias Wilms1, Paula Ludovico2, Joris Winderickx1
In this article, Deprez et al. discuss accumulating evidence indicates that pH homeostasis plays a prominent role in the determination of ageing and longevity, thereby providing new perspectives and avenues to explore the underlying molecular mechanisms.
Guidelines and recommendations on yeast cell death nomenclature
Didac Carmona-Gutierrez1,‡,*, Maria Anna Bauer1,‡, Andreas Zimmermann1, Andrés Aguilera2, Nicanor Austriaco3, Kathryn Ayscough4, Rena Balzan5, Shoshana Bar-Nun6, Antonio Barrientos7,8, Peter Belenky9, Marc Blondel10, Ralf J. Braun11, Michael Breitenbach12, William C. Burhans13, Sabrina Büttner1,14, Duccio Cavalieri15, Michael Chang16, Katrina F. Cooper17, Manuela Côrte-Real18, Vítor Costa19–21, Christophe Cullin22, Ian Dawes23, Jörn Dengjel24, Martin B. Dickman25, Tobias Eisenberg1,26, Birthe Fahrenkrog27, Nicolas Fasel28, Kai-Uwe Fröhlich1, Ali Gargouri29, Sergio Giannattasio30, Paola Goffrini31, Campbell W. Gourlay32, Chris M. Grant33, Michael T. Greenwood34, Nicoletta Guaragnella30, Thomas Heger35, Jürgen Heinisch36, Eva Herker37, Johannes M. Herrmann38, Sebastian Hofer1, Antonio Jiménez-Ruiz39, Helmut Jungwirth1, Katharina Kainz1, Dimitrios P. Kontoyiannis40, Paula Ludovico41,42, Stéphen Manon43, Enzo Martegani44, Cristina Mazzoni45, Lynn A. Megeney46–48, Chris Meisinger49, Jens Nielsen50–52, Thomas Nyström53, Heinz D. Osiewacz54, Tiago F. Outeiro55–58, Hay-Oak Park59, Tobias Pendl1, Dina Petranovic50,51, Stephane Picot60,61, Peter Polčic62, Ted Powers63, Mark Ramsdale64, Mark Rinnerthaler65, Patrick Rockenfeller1,32, Christoph Ruckenstuhl1, Raffael Schaffrath66, Maria Segovia67, Fedor F. Severin68, Amir Sharon69, Stephan J. Sigrist70, Cornelia Sommer-Ruck1, Maria João Sousa18, Johan M. Thevelein71,72, Karin Thevissen73, Vladimir Titorenko74, Michel B. Toledano75, Mick Tuite32, F.-Nora Vögtle49, Benedikt Westermann11, Joris Winderickx76, Silke Wissing77, Stefan Wölfl78, Zhaojie J. Zhang79, Richard Y. Zhao80, Bing Zhou81, Lorenzo Galluzzi82–84,*, Guido Kroemer84–90,*, Frank Madeo1,26,*
In this review, we propose unified criteria for the definition of accidental, regulated, and programmed forms of cell death in yeast based on a series of morphological and biochemical criteria. Specifically, we provide consensus guidelines on the differential definition of terms including apoptosis, regulated necrosis, and autophagic cell death, as we refer to additional cell death routines that are relevant for the biology of yeast.
Burkholderia gladioli strain NGJ1 deploys a prophage tail-like protein for mycophagy
Rahul Kumar1, Sunil Kumar Yadav1, Durga Madhab Swain1 and Gopaljee Jha1
In this article, the authors comment on the study “A prophage tail-like protein is deployed by Burkholderia bacteria to feed on fungi” by Swain et al. (Nature Communications, 2017), discussing that a prophage tail-like protein (Bg_9562) is essential for mycophagy. The protein may help the bacteria to survive in certain ecological niches and, considering its broad-spectrum antifungal activity, may be potentially useful in biotechnological applications to control fungal diseases.
Targeting GATA transcription factors – a novel strategy for anti-aging interventions?
Andreas Zimmermann1, Katharina Kainz1,2, Sebastian J. Hofer1,3, Maria A. Bauer1, Sabrina Schroeder1, Jörn Dengjel4, Federico Pietrocola5, Oliver Kepp6-9, Christoph Ruckenstuhl1, Tobias Eisenberg1,3,10,11, Stephan J. Sigrist12, Frank Madeo1,3,10, Guido Kroemer6-9, 13-15 and Didac Carmona-Gutierrez1
This article comments on work published by Carmona-Gutierrez et al. (Nat Commun., 2019), which identified a natural compound, 4,4′-dimethoxychalcone, inducing autophagy and prolonging lifespan in different organisms through a mechanism that involves GATA transcription factors.
In the beginning was the word: How terminology drives our understanding of endosymbiotic organelles
Miroslav Oborník 1,2
This In the Pit article argues that the naming conventions for biological entities influence research perspectives and methodologies, advocating for mitochondria and plastids to be classified and named as bacteria due to their endosymbiotic origins, with potential implications for our understanding of bacterial prevalence, definitions of the microbiome and multicellularity, and the concept of endosymbiotic domestication.
What’s in a name? How organelles of endosymbiotic origin can be distinguished from endosymbionts
Ansgar Gruber1
This In the Pit article suggests redefining the relationship between hosts and endosymbionts, like mitochondria and plastids, as a single species based on “sexual symbiont integration,” the loss of independent speciation, and congruence in genetic recombination and population sizes, rather than solely on historic classifications or structural properties.
Microbial wars: competition in ecological niches and within the microbiome
Maria A. Bauer1, Katharina Kainz1, Didac Carmona-Gutierrez1 and Frank Madeo1,2
In this Editorial Bauer et al. provide a brief overview on microbial competition and discuss some of its roles and consequences that directly affect humans.
Exploring the mechanism of amebic trogocytosis: the role of amebic lysosomes
Allissia A. Gilmartin1 and William A. Petri, Jr1,2,3
In this article, the authors comment on the study “Inhibition of Amebic Lysosomal Acidification Blocks Amebic Trogocytosis and Cell Killing” by Gilmartin et al. (MBio, 2017), discussing the the role of amebic lysosomes in Trogocytosis, the intracellular transfer of fragments of cell material.
Uncovering the hidden: complexity and strategies for diagnosing latent tuberculosis
Mario Alberto Flores-Valdez
This editorial postulates that advanced proteomic and transcriptomic techniques are evolving and may enhance the detection of latent tuberculosis, thereby distinguishing true M. tuberculosis infections from other conditions, which is vital for controlling potential reactivation and transmission.
The Yin & Yang of Mitochondrial Architecture – Interplay of MICOS and F1Fo-ATP synthase in cristae formation
Heike Rampelt1 and Martin van der Laan2
This Editorial posits that mitochondrial cristae architecture is shaped by the interplay of MICOS and ATP synthase, with a recent study illuminating their roles in cristae formation and maintenance.
When a ribosomal protein grows up – the ribosome assembly path of Rps3
Brigitte Pertschy
This article comments on two papers by Mitterer et al., which followed yeast protein Rps3, highlighting the sophisticated mechanisms for protein protection, nuclear transport, and integration into pre-ribosomal particles for final assembly with 40S subunits.
Microbial Cell
is an open-access, peer-reviewed journal that publishes exceptionally relevant research works that implement the use of unicellular organisms (and multicellular microorganisms) to understand cellular responses to internal and external stimuli and/or human diseases.
you can trust
Can’t find what you’re looking for?
You can browse all our issues and published articles here.
FAQs
Peer-reviewed, open-access research using unicellular organisms (and multicellular microorganisms) to understand cellular responses and human disease.
The journal (founded in 2014) is led by its Editors-in-Chief Frank Madeo, Didac Carmona-Gutierrez, and Guido Kroemer
Microbial Cell has been publishing original scientific literature since 2014, and from the very beginning has been managed by active scientists through an independent Publishing House (Shared science Publishers). The journal was conceived as a platform to acknowledge the importance of unicellular organisms, both as model systems as well as in the biological context of human health and disease.
Ever since, Microbial Cell has very positively developed and strongly grown into a respected journal in the unicellular research community and even beyond. This scientific impact is reflected in the yearly number of citations obtained by articles published in Microbial Cell, as recorded by the Web of Science (Clarivate, formerly Thomson/Reuters):
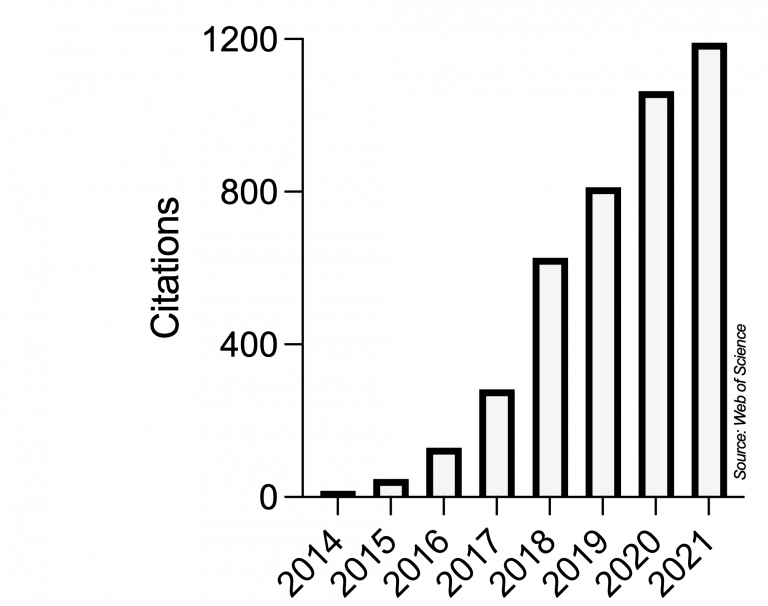
The scientific impact of Microbial Cell is also mirrored in a series of milestones:
2015: Microbial Cell is included in the Emerging Sources Citation Index (ESCI), a selection of developing journals drafted by Clarivate Analytics based on the candidate’s publishing standards, quality, editorial content, and citation data. Note: As an ESCI-selected journal, Microbial Cell is currently being evaluated in a rigorous and long process to determine an inclusion in the Science Citation Index Expanded (SCIE), which allows the official calculation of Clarivate Analytics’ impact factor.
2016: Microbial Cell is awarded the so-called DOAJ Seal by the selective Directory of Open Access Journals (DOAJ). The DOAJ Seal is an exclusive mark of certification for open access journals granted by DOAJ to journals that adhere to outstanding best practice and achieve an extra high and clear commitment to open access and high publishing standards.
2017: Microbial Cell is included in Pubmed Central (PMC), allowing the archiving of all the journal’s articles in PMC and PubMed.
2019: Microbial Cell is indexed in the prestigious abstract and citation database Scopus after a thorough selection process. This also means that Microbial Cell obtains, for the first time, an official Scopus CiteScore as well as an official journal ranking in the Scimago Journal and Country Ranking.
2022: Microbial Cell’s CiteScore reaches a value of 7.2 for the year 2021, positioning Microbial Cell among the top microbiology journals (previously available CiteScores: 2019: 5.4; 2020: 5.1).
2022: Microbial Cell is indexed in the highly selective Science Citation Index Expanded™, which covers approx. 9,500 of the world’s most impactful journals across 178 scientific disciplines. In their journal selection and curation process, Clarivate´s editors apply 24 ‘quality’ criteria and four ‘impact’ criteria to select the most influential journals in their respective fields. This selection is also a pre-requisite for inclusion in the JCR, which features the impact factor.
2022: Microbial Cell is listed in the Journal Citation Reports™ (JCR), and obtains its first official Journal Impact Factor™ (JIF) for the year 2021: 5.316.Check Article Types and Manuscript Preparation guidelines. Submit online via Scholastica.

Sulfur dioxide resistance in Saccharomyces cerevisiae: beyond SSU1
Estéfani García-Ríos1 and José Manuel Guillamón1
This article discusses the importance of understanding sulfite resistance in Saccharomyces cerevisiae due to its use in winemaking and the potential role of the transcription factor Com2. While the SSU1 gene and its activity have been correlated with sulfite tolerance, the work by Lage et al. (2019) indicates that Com2 might control a large percentage of the genes activated by SO2 and contribute to the yeast’s protective response, offering new insights into the molecular factors influencing this oenological trait.