
Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.

Luminal acetylation of microtubules is not essential for Plasmodium berghei and Toxoplasma gondii survival
Acetylation of α-tubulin at lysine 40 is not essential for cytoskeletal stability in Plasmodium berghei or Toxoplasma gondii, suggesting redundancy and plasticity in microtubule regulation in these parasites.

The dual-site agonist for human M2 muscarinic receptors Iper-8-naphtalimide induces mitochondrial dysfunction in Saccharomyces cerevisiae
S. cerevisiae is a model to study human GPCRs. N-8-Iper, active against glioblastoma via M2 receptor, causes mitochondrial damage in yeast by binding Ste2, highlighting evolutionary conservation of GPCRs.

Integrative Omics reveals changes in the cellular landscape of peroxisome-deficient pex3 yeast cells
To uncover the consequences of peroxisome deficiency, we compared Saccharomyces cerevisiae wild-type with pex3 cells, which lack peroxisomes, employing quantitative proteomics and transcriptomics technologies.

Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
Rebekkah E. Pope1, Patrick Ballmann2, Lisa Whitworth3 and Rolf A. Prade1,*
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
Evelyn Tevere1,a, María G. Mediavilla1,a, Cecilia B. Di Capua1, Marcelo L. Merli1, Carlos Robello2,3, Luisa Berná2,4 and Julia A. Cricco
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.
Sir2 regulates selective autophagy in stationary-phase yeast cells
Ji-In Ryua, Juhye Junga, and Jeong-Yoon Kim
This study establishes Sir2 as a previously unrecognized regulator of selective autophagy during the stationary phase and highlight how cells dynamically control organelle degradation.
Protein oxidation in the intermembrane space of mitochondria is substrate-specific rather than general
Valentina Peleh1, Jan Riemer2, Andrew Dancis3 and Johannes M. Herrmann1
In this work, the authors suggest that in Saccharomyces cerevisiae, the Mia40-dependent oxidation of proteins in the intermembrane space only takes place in specific proteins and presumably relies on the presence of Mia40-binding sites.
Deletion of AIF1 but not of YCA1/MCA1 protects Saccharomyces cerevisiae and Candida albicans cells from caspofungin-induced programmed cell death
Christopher Chin1,2,#, Faith Donaghey1,#, Katherine Helming1,3,#, Morgan McCarthy1,#, Stephen Rogers1, and Nicanor Austriaco1
This work suggests that deleting AIF1 but not YCA1/MCA1 protects S. cerevisiae and Candida albicans from caspofungin-induced cell death. This is not only the first time that AIF1 has been specifically tied to cell death in Candida but also the first time that caspofungin resistance has been linked to the cell death machinery in yeast.
Reduced TORC1 signaling abolishes mitochondrial dysfunctions and shortened chronological lifespan of Isc1p-deficient cells
Vitor Teixeira1,2, Tânia C. Medeiros1, Rita Vilaça1,2, Pedro Moradas-Ferreira1,2, and Vítor Costa1,2
Overall, this article shows that the TORC1-Sch9p axis is deregulated in Isc1p-deficient Saccharomyces cerevisiae cells, contributing to mitochondrial dysfunction, enhanced oxidative stress sensitivity and premature aging of isc1Δ cells.
Early manifestations of replicative aging in the yeast Saccharomyces cerevisiae.
Maksim I. Sorokin1,3, Dmitry A. Knorre2,3, and Fedor F. Severin2,3
The data preseted herein suggest that retrograde signaling starts to malfunction in relatively young cells, leading to accumulation of heterogeneous mitochondria within one cell. The latter may further contribute to a decline in stress resistances.
Tracking autophagy during proliferation and differentiation of Trypanosoma brucei
William R. Proto1, Nathaniel G. Jones1, Graham H. Coombs2, and Jeremy C. Mottram1
This article provides insights into the function of autophagy, a cellular degradation and recycling pathway, in the protozoan parasite Trypanosoma brucei.
A pseudokinase couples signaling pathways to enable asymmetric cell division in a bacterium
W. Seth Childers and Lucy Shapiro
In this article, the authors comment on the study “Cell fate regulation governed by a repurposed bacterial histidine kinase” by Childers et al., PLoS Biol. 2014 Oct 28;12(10):e1001979.
Targeting of chromatin readers: a novel strategy used by the Shigella flexneri virulence effector OspF to reprogram transcription
Habiba Harouz, Christophe Rachez, Benoit Meijer, Christian Muchardt, Laurence Arbibe.
In this microreview, the authors discuss the article “Shigella flexneri targets the HP1γ subcode through the phosphothreoninelyase OspF” by Harouz et al. (2014), EMBO J, 22 : 2606-2622.
Plasmodium spp. membrane glutathione S-transferases: detoxification units and drug targets
Andreas Martin Lisewski
This article comments on work published by Lisewski et al. (Cell, 2014), which reported the first examples of membrane-associated proteins in eicosanoid and glutathione metabolism members among Plasmodium spp.
Proline cis-trans isomerization is influenced by local lysine acetylation-deacetylation
Françoise S. Howe and Jane Mellor
This article comments on work published by Howe et al. (Mol Cell, 2014), which shows that local lysine acetylation and deacetylation modulate proline cis-trans isomerization in Saccharomyces cerevisiae.
On the link between cell cycle and infection of the Alphaproteobacterium Brucella abortus
Michaël Deghelt, Jean-Jacques Letesson, Xavier De Bolle
This article comments on work published by Deghelt et al. (Nat Comm, 2014), which describe a cell cycle arrest and resume during the Brucella abortus trafficking in host cell, suggesting that like the model Alphaproteobacterium Caulobacter crescentus, these bacteria are able to block their cell cycle at the G1 phase when starvation is sensed.
Divide and conquer: processive transport enables multidrug transporters to tackle challenging drugs
Nir Fluman and Eitan Bibi
This article comments on work published by Fluman et al. (Nat Comm, 2014), which describes the ability of bacterial multidrug transporters to move long molecules through the membrane in a processive manner.
The dual role of cyclin C connects stress regulated gene expression to mitochondrial dynamics
Randy Strich and Katrina F. Cooper
This work summarizes the role cyclin C plays in regulating stress-responsive transcription in the budding yeast Saccharomyces cerevisiae, including mitochondrial fission and regulated cell death.
Combinatorial stress responses: direct coupling of two major stress responses in Escherichia coli
Daniel R. Brown, Geraint Barton, Zhensheng Pan, Martin Buck and Sivaramesh Wigneshweraraj
This article comments on work published by Brown et al. (Nat Comm, 2014), which showed that the transcription of relA is activated by NtrC during nitrogen starvation, revealing that in E. coli and related bacteria, NtrC functions in combinatorial stress and serves to couple two major stress responses, the Ntr response and stringent response.
The replication timing program in the hands of two HDACs
Kazumasa Yoshida1,2, Armelle Lengronne1 and Philippe Pasero1
This article comments on work published by Yoshida et al. (Mol Cell, 2014), which performed a systematic analysis of the role of histone deacetylases (HDACs) in the regulation of origin activity in budding yeast, finding that the epigenetic regulation of repetitive sequences is a key determinant of the DNA replication program.
Starting with a degron: N-terminal formyl-methionine of nascent bacterial proteins contributes to their proteolytic control
R. Jürgen Dohmen
In this article, the author comments on the study “Formyl-methionine as a degradation signal at the N-termini of bacterial proteins.” by Piatkov et al. (Microbial Cell, 2015), discussing a novel N-terminal degradation signal (N-degron) that targets nascent proteins for degradation in Escherichia coli by a new branch of the bacterial N-end rule pathway, termed the fMet/N-end rule pathway
Elongation factor-P at the crossroads of the host-endosymbiont interface
Andrei Rajkovic1, Anne Witzky2, William Navarre3, Andrew J. Darwin4 and Michael Ibba5
Elongation factor P (EF-P) is an ancient bacterial translational factor that aids the ribosome in polymerizing oligo-prolines. EF-P structurally resembles tRNA and binds in-between the exit and peptidyl sites of the ribosome to accelerate the intrinsically slow reaction of peptidyl-prolyl bond formation. Recent studies have identified in separate organisms, two evolutionarily convergent EF-P post-translational modification systems (EPMS), split predominantly between gammaproteobacteria, and betaproteobacteria. Here, the authors highlight the recent discoveries made regarding EPMSs, with a focus on how these incomplete modification pathways shape or have been shaped by the endosymbiont-host relationship.
Feelin’ it: Differential oxidative stress sensing mediated by Cyclin C
W. Scott Moye-Rowley
Microbial cells that live exposed directly to their environmental milieu are faced with the challenge of adapting to the dynamic stress conditions that will inevitably be encountered. These stress conditions may vary over wide ranges and the most efficient responses would be tuned to produce a proportional buffering change. A mild stress would most efficiently be dealt with by a mild metabolic reprogramming that would prevent serious damage. A more severe environmental challenge would demand a more dramatic cellular compensatory response.
Subverting lysosomal function in Trypanosoma brucei
Sam Alsford
This article discusses Koh et al. (2015) “The lysosomotropic drug LeuLeu-OMe induces lysosome disruption and autophagy-independent cell death in Trypanosoma brucei (Microbial Cell 2(8): 288-298).
Entamoeba histolytica – tumor necrosis factor: a fatal attraction
Serge Ankri
This article comments on the study “In Entamoeba histolytica, a BspA family protein is required for chemotaxis toward tumour necrosis factor” by Silvestre et al. (Microbial Cell, 2015).
Toxoplasma control of host apoptosis: the art of not biting too hard the hand that feeds you
Sébastien Besteiro
Toxoplasma gondii is an obligate intracellular parasite that is able to infect a multitude of different vertebrate hosts and can survive in virtually any nucleated cell. Here, the authors discuss the article “Toxoplasma gondii inhibits cytochrome c-induced caspase activation in its host cell by interference with holo-apoptosome assembly” by Graumann et al. (2015, Microbial Cell).
A safety catch for ornithine decarboxylase degradation
Christof Taxis
Feedback inhibition is a common mechanism to adjust the activity of an enzyme in accordance with the abundance of a product. This article comments on the study “Polyamines directly promote antizyme-mediated degradation of ornithine decarboxylase by the proteasome” by Beenukumar et al. (2015), Microbial Cell.
Fancy a gene? A surprisingly complex evolutionary history of peroxiredoxins.
Alena Zíková1,2, Miroslav Oborník1,2,3 and Julius Lukeš1,2,4
In this comment, the authors discuss the article “Prokaryotic ancestry and gene fusion of a dual localized peroxiredoxin in malaria parasites” (Djuika et al., Microbial Cell 2015).
Quorum protection, growth and survival
Ian G . Macreadie
For the growth of a cell culture, one inoculates not with one cell but with a quorum of cells. This most often a requirement, not just a convenience, and most of us take this for granted without question. Here this observation is re-examined to understand why a quorum may be required to grow cells. The importance of quorums may be widespread in the aspects of microbiology they affect. It is very likely that quorums are connected with and have a large impact on the determination of Minimal Inhibitory Concentrations. It is also possible that low cell density may adversely affect cell survival, however, this is an area where even less is known. The need for a quorum might affect other aspects of microbial cell culture, cell isolation and cell preservation. Effects also extend to mammalian cell culture. Here I seek to review studies that have been documented and speculate on how the information might be utilized in the future.
Microbial Cell
is an open-access, peer-reviewed journal that publishes exceptionally relevant research works that implement the use of unicellular organisms (and multicellular microorganisms) to understand cellular responses to internal and external stimuli and/or human diseases.
you can trust
Can’t find what you’re looking for?
You can browse all our issues and published articles here.
FAQs
Peer-reviewed, open-access research using unicellular organisms (and multicellular microorganisms) to understand cellular responses and human disease.
The journal (founded in 2014) is led by its Editors-in-Chief Frank Madeo, Didac Carmona-Gutierrez, and Guido Kroemer
Microbial Cell has been publishing original scientific literature since 2014, and from the very beginning has been managed by active scientists through an independent Publishing House (Shared science Publishers). The journal was conceived as a platform to acknowledge the importance of unicellular organisms, both as model systems as well as in the biological context of human health and disease.
Ever since, Microbial Cell has very positively developed and strongly grown into a respected journal in the unicellular research community and even beyond. This scientific impact is reflected in the yearly number of citations obtained by articles published in Microbial Cell, as recorded by the Web of Science (Clarivate, formerly Thomson/Reuters):
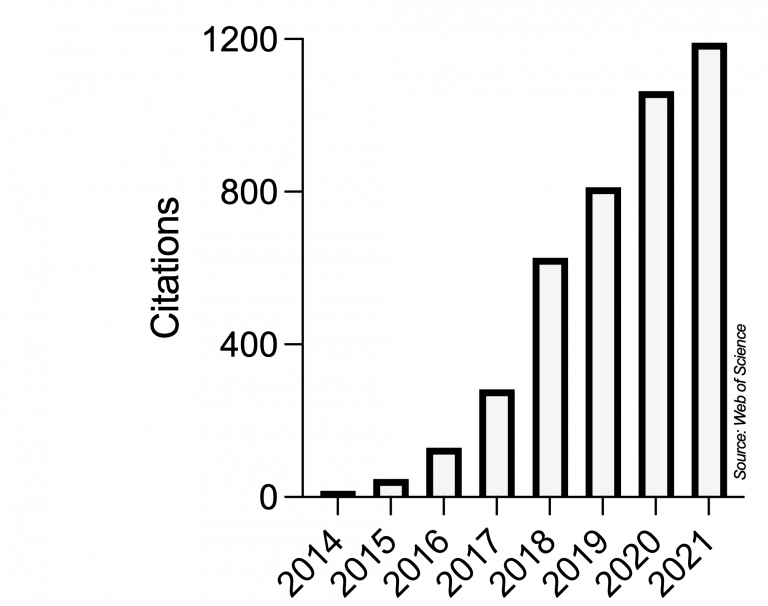
The scientific impact of Microbial Cell is also mirrored in a series of milestones:
2015: Microbial Cell is included in the Emerging Sources Citation Index (ESCI), a selection of developing journals drafted by Clarivate Analytics based on the candidate’s publishing standards, quality, editorial content, and citation data. Note: As an ESCI-selected journal, Microbial Cell is currently being evaluated in a rigorous and long process to determine an inclusion in the Science Citation Index Expanded (SCIE), which allows the official calculation of Clarivate Analytics’ impact factor.
2016: Microbial Cell is awarded the so-called DOAJ Seal by the selective Directory of Open Access Journals (DOAJ). The DOAJ Seal is an exclusive mark of certification for open access journals granted by DOAJ to journals that adhere to outstanding best practice and achieve an extra high and clear commitment to open access and high publishing standards.
2017: Microbial Cell is included in Pubmed Central (PMC), allowing the archiving of all the journal’s articles in PMC and PubMed.
2019: Microbial Cell is indexed in the prestigious abstract and citation database Scopus after a thorough selection process. This also means that Microbial Cell obtains, for the first time, an official Scopus CiteScore as well as an official journal ranking in the Scimago Journal and Country Ranking.
2022: Microbial Cell’s CiteScore reaches a value of 7.2 for the year 2021, positioning Microbial Cell among the top microbiology journals (previously available CiteScores: 2019: 5.4; 2020: 5.1).
2022: Microbial Cell is indexed in the highly selective Science Citation Index Expanded™, which covers approx. 9,500 of the world’s most impactful journals across 178 scientific disciplines. In their journal selection and curation process, Clarivate´s editors apply 24 ‘quality’ criteria and four ‘impact’ criteria to select the most influential journals in their respective fields. This selection is also a pre-requisite for inclusion in the JCR, which features the impact factor.
2022: Microbial Cell is listed in the Journal Citation Reports™ (JCR), and obtains its first official Journal Impact Factor™ (JIF) for the year 2021: 5.316.Check Article Types and Manuscript Preparation guidelines. Submit online via Scholastica.
