
Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.

Luminal acetylation of microtubules is not essential for Plasmodium berghei and Toxoplasma gondii survival
Acetylation of α-tubulin at lysine 40 is not essential for cytoskeletal stability in Plasmodium berghei or Toxoplasma gondii, suggesting redundancy and plasticity in microtubule regulation in these parasites.

The dual-site agonist for human M2 muscarinic receptors Iper-8-naphtalimide induces mitochondrial dysfunction in Saccharomyces cerevisiae
S. cerevisiae is a model to study human GPCRs. N-8-Iper, active against glioblastoma via M2 receptor, causes mitochondrial damage in yeast by binding Ste2, highlighting evolutionary conservation of GPCRs.

Integrative Omics reveals changes in the cellular landscape of peroxisome-deficient pex3 yeast cells
To uncover the consequences of peroxisome deficiency, we compared Saccharomyces cerevisiae wild-type with pex3 cells, which lack peroxisomes, employing quantitative proteomics and transcriptomics technologies.

Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
Rebekkah E. Pope1, Patrick Ballmann2, Lisa Whitworth3 and Rolf A. Prade1,*
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
Evelyn Tevere1,a, María G. Mediavilla1,a, Cecilia B. Di Capua1, Marcelo L. Merli1, Carlos Robello2,3, Luisa Berná2,4 and Julia A. Cricco
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.
Sir2 regulates selective autophagy in stationary-phase yeast cells
Ji-In Ryua, Juhye Junga, and Jeong-Yoon Kim
This study establishes Sir2 as a previously unrecognized regulator of selective autophagy during the stationary phase and highlight how cells dynamically control organelle degradation.
Protein oxidation in the intermembrane space of mitochondria is substrate-specific rather than general
Valentina Peleh1, Jan Riemer2, Andrew Dancis3 and Johannes M. Herrmann1
In this work, the authors suggest that in Saccharomyces cerevisiae, the Mia40-dependent oxidation of proteins in the intermembrane space only takes place in specific proteins and presumably relies on the presence of Mia40-binding sites.
Deletion of AIF1 but not of YCA1/MCA1 protects Saccharomyces cerevisiae and Candida albicans cells from caspofungin-induced programmed cell death
Christopher Chin1,2,#, Faith Donaghey1,#, Katherine Helming1,3,#, Morgan McCarthy1,#, Stephen Rogers1, and Nicanor Austriaco1
This work suggests that deleting AIF1 but not YCA1/MCA1 protects S. cerevisiae and Candida albicans from caspofungin-induced cell death. This is not only the first time that AIF1 has been specifically tied to cell death in Candida but also the first time that caspofungin resistance has been linked to the cell death machinery in yeast.
Reduced TORC1 signaling abolishes mitochondrial dysfunctions and shortened chronological lifespan of Isc1p-deficient cells
Vitor Teixeira1,2, Tânia C. Medeiros1, Rita Vilaça1,2, Pedro Moradas-Ferreira1,2, and Vítor Costa1,2
Overall, this article shows that the TORC1-Sch9p axis is deregulated in Isc1p-deficient Saccharomyces cerevisiae cells, contributing to mitochondrial dysfunction, enhanced oxidative stress sensitivity and premature aging of isc1Δ cells.
Early manifestations of replicative aging in the yeast Saccharomyces cerevisiae.
Maksim I. Sorokin1,3, Dmitry A. Knorre2,3, and Fedor F. Severin2,3
The data preseted herein suggest that retrograde signaling starts to malfunction in relatively young cells, leading to accumulation of heterogeneous mitochondria within one cell. The latter may further contribute to a decline in stress resistances.
Tracking autophagy during proliferation and differentiation of Trypanosoma brucei
William R. Proto1, Nathaniel G. Jones1, Graham H. Coombs2, and Jeremy C. Mottram1
This article provides insights into the function of autophagy, a cellular degradation and recycling pathway, in the protozoan parasite Trypanosoma brucei.
From the Uncharacterized Protein Family 0016 to the GDT1 family: Molecular insights into a newly-characterized family of cation secondary transporters
Louise Thines1, Jiri Stribny1 and Pierre Morsomme1
This review outlines how the formerly uncharacterized UPF0016 family, now known as the Gdt1 family, plays key roles in cation transport – especially Mn²⁺ – across species from bacteria to humans. These proteins are crucial for processes like glycosylation, photosynthesis, and calcium signaling, with functions linked to their localization in membranes such as the Golgi, chloroplast, and plasma membrane and by that highlighting their evolutionary conservation and physiological relevance, offering insights into their shared and distinct features across organisms.
A broad-spectrum antibiotic adjuvant SLAP-S25: one stone many birds
Meirong Song1 and Kui Zhu1
This article refers to the study “A broad-spectrum antibiotic adjuvant reverses multidrug-resistant Gram-negative pathogens” by Song et al. (Nat Microbiol, 2020), which deals with the growing threat of antibiotic resistance, with few new drugs being developed for decades. The study found that the peptide SLAP-S25 enhances the efficacy of several antibiotics against resistant Gram-negative bacteria by disrupting their membranes, thereby increasing drug uptake. This suggests that bacterial membranes are promising targets for new antibiotic adjuvants.
Hiding in plain sight: vesicle-mediated export and transmission of prion-like proteins
Mehdi Kabani1
This article relates to the study “Glucose availability dictates the export of the soluble and prion forms of Sup35p via periplasmic or extracellular vesicles” by Kabani et al. (Mol Microbiol, 2020) that provides compelling evidence that yeast prions, such as Sup35p in its infectious [PSI⁺] state, can be exported via both extracellular vesicles (EVs) and periplasmic vesicles (PVs), with this export being modulated by environmental glucose levels. The discovery that prion particles are released in high amounts through PVs during glucose starvation adds a new dimension to our understanding of prion transmission and opens up fascinating possibilities for exploring vesicle-mediated spread of protein aggregates in neurodegenerative diseases using yeast as a model system.
Regulation of Cdc42 for polarized growth in budding yeast
Kristi E. Miller1,2, Pil Jung Kang1 and Hay-Oak Park1
This review highlights how studies in budding yeast have revealed a biphasic mechanism of Cdc42 activation that governs cell polarity establishment, with implications for understanding similar processes in mammalian cells and the role of Cdc42 in aging.
Yeast-based assays for the functional characterization of cancer-associated variants of human DNA repair genes
Tiziana Cervelli1, Samuele Lodovichi1, Francesca Bellè1 and Alvaro Galli1
This article highlights how the genetic tractability and conserved DNA repair pathways of yeast make it a powerful system for functionally characterizing human cancer-associated variants in DNA repair genes, aiding in risk assessment and therapeutic decision-making.
A novel c-di-GMP signal system regulates biofilm formation in Pseudomonas aeruginosa
Gukui Chen1 and Haihua Liang1
This article relates to the study “The SiaA/B/C/D signaling network regulates biofilm formation in Pseudomonas aeruginosa” by Chen et al. (EMBO J, 2020) that reveals a novel signaling network encoded by the siaABCD operon in Pseudomonas aeruginosa that regulates biofilm and aggregate formation by controlling the diguanylate cyclase activity of SiaD through phosphorylation-dependent interactions with SiaC, highlighting a potential antimicrobial target.
Regulation of anti-microbial autophagy by factors of the complement system
Christophe Viret1, Aurore Rozières1, Rémi Duclaux-Loras1, Gilles Boschetti1, Stéphane Nancey1 and
Mathias Faure1,2
This review explores emerging evidence that components of the complement system, beyond their traditional immune roles, modulate autophagy – particularly xenophagy – thereby influencing cell-autonomous antimicrobial responses during host-pathogen interactions.
More than flipping the lid: Cdc50 contributes to echinocandin resistance by regulating calcium homeostasis in Cryptococcus neoformans
Chengjun Cao1 and Chaoyang Xue1,2
In this article, the authors comment on the study “A mechanosensitive channel governs lipid flippase-mediated echinocandin resistance in Cryptococcus neoformans” by Cao et al. (mBio, 2019), which uncovers a dual role for the lipid flippase subunit Cdc50 in Cryptococcus neoformans, linking lipid translocation and calcium signaling via its interaction with the mechanosensitive channel Crm1, thereby contributing to innate resistance against the antifungal drug caspofungin.
Non-genetic impact factors on chronological lifespan and stress resistance of baker’s yeast
Michael Sauer and Diethard Mattanovich
This article comments on work published by Bisschops et al. (Microbial Cell, 2015), which illustrates how important the choice of the experimental setup is and how culture conditions influcence cellular aging and survival in biotechnological processes.
What’s old is new again: yeast mutant screens in the era of pooled segregant analysis by genome sequencing
Chris Curtin and Toni Cordente
This article comments on work published by Den Abt et al. (Microbial Cell, 2016), which identified genes involved in ethyl acetate formation in a yeast mutant screen based on a new approach combining repeated rounds of chemical mutagenesis and pooled segregant analysis by whole genome sequencing.
The complexities of bacterial-fungal interactions in the mammalian gastrointestinal tract
Eduardo Lopez-Medina1 and Andrew Y. Koh2
This article comments on work published by Lopez-Medina et al. (PLoS Pathog, 2015) and Fan et al. (Nat Med, 2015), which utilize an “artificial” niche, the antibiotic-treated gut with concomitant pathogenic microbe expansion, to gain insight in bacterial-fungal interactions in clinically common scenarios.
Gearing up for survival – HSP-containing granules accumulate in quiescent cells and promote survival
Ruofan Yu and Weiwei Dang
This article comments on work published by Lee et al. (Microbial Cell, 2016), which reports that distinct granules are formed in quiescent and non-quiescent cells, which determines their respective cell fates.
Yeast screening platform identifies FDA-approved drugs that reduce Aβ oligomerization
Triana Amen1,2 and Daniel Kaganovich1
This article comments on work published by Park et al. (Microbial Cell, 2016), which discovered a number of small molecules capable of modulating Aβ aggregation in a yeast model.
Groupthink: chromosomal clustering during transcriptional memory
Kevin A. Morano
In this article, the authors comment on the study “NO1 transcriptional memory leads to DNA zip code-dependent interchromosomal clustering.” by Brickner et al. (Microbial Cell, 2015), discussing the importance and molecular mechanisms of chromosomal clustering during transcriptional memory.
Yeast proteinopathy models: a robust tool for deciphering the basis of neurodegeneration
Amit Shrestha1, 2 and Lynn A. Megeney1, 2, 3
Protein quality control or proteostasis is an essential determinant of basic cell health and aging. Eukaryotic cells have evolved a number of proteostatic mechanisms to ensure that proteins retain functional conformation, or are rapidly degraded when proteins misfold or self-aggregate. This article discusses the use of budding yeast as a robust proxy to study the intersection between proteostasis and neurodegenerative disease.
Microbial Cell
is an open-access, peer-reviewed journal that publishes exceptionally relevant research works that implement the use of unicellular organisms (and multicellular microorganisms) to understand cellular responses to internal and external stimuli and/or human diseases.
you can trust
Can’t find what you’re looking for?
You can browse all our issues and published articles here.
FAQs
Peer-reviewed, open-access research using unicellular organisms (and multicellular microorganisms) to understand cellular responses and human disease.
The journal (founded in 2014) is led by its Editors-in-Chief Frank Madeo, Didac Carmona-Gutierrez, and Guido Kroemer
Microbial Cell has been publishing original scientific literature since 2014, and from the very beginning has been managed by active scientists through an independent Publishing House (Shared science Publishers). The journal was conceived as a platform to acknowledge the importance of unicellular organisms, both as model systems as well as in the biological context of human health and disease.
Ever since, Microbial Cell has very positively developed and strongly grown into a respected journal in the unicellular research community and even beyond. This scientific impact is reflected in the yearly number of citations obtained by articles published in Microbial Cell, as recorded by the Web of Science (Clarivate, formerly Thomson/Reuters):
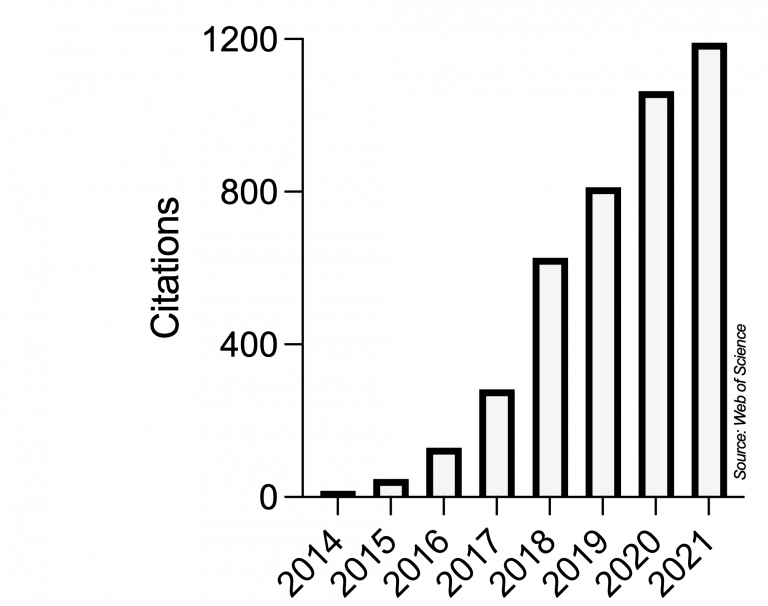
The scientific impact of Microbial Cell is also mirrored in a series of milestones:
2015: Microbial Cell is included in the Emerging Sources Citation Index (ESCI), a selection of developing journals drafted by Clarivate Analytics based on the candidate’s publishing standards, quality, editorial content, and citation data. Note: As an ESCI-selected journal, Microbial Cell is currently being evaluated in a rigorous and long process to determine an inclusion in the Science Citation Index Expanded (SCIE), which allows the official calculation of Clarivate Analytics’ impact factor.
2016: Microbial Cell is awarded the so-called DOAJ Seal by the selective Directory of Open Access Journals (DOAJ). The DOAJ Seal is an exclusive mark of certification for open access journals granted by DOAJ to journals that adhere to outstanding best practice and achieve an extra high and clear commitment to open access and high publishing standards.
2017: Microbial Cell is included in Pubmed Central (PMC), allowing the archiving of all the journal’s articles in PMC and PubMed.
2019: Microbial Cell is indexed in the prestigious abstract and citation database Scopus after a thorough selection process. This also means that Microbial Cell obtains, for the first time, an official Scopus CiteScore as well as an official journal ranking in the Scimago Journal and Country Ranking.
2022: Microbial Cell’s CiteScore reaches a value of 7.2 for the year 2021, positioning Microbial Cell among the top microbiology journals (previously available CiteScores: 2019: 5.4; 2020: 5.1).
2022: Microbial Cell is indexed in the highly selective Science Citation Index Expanded™, which covers approx. 9,500 of the world’s most impactful journals across 178 scientific disciplines. In their journal selection and curation process, Clarivate´s editors apply 24 ‘quality’ criteria and four ‘impact’ criteria to select the most influential journals in their respective fields. This selection is also a pre-requisite for inclusion in the JCR, which features the impact factor.
2022: Microbial Cell is listed in the Journal Citation Reports™ (JCR), and obtains its first official Journal Impact Factor™ (JIF) for the year 2021: 5.316.Check Article Types and Manuscript Preparation guidelines. Submit online via Scholastica.

Similar environments but diverse fates: Responses of budding yeast to nutrient deprivation.
Saul M. Honigberg
Diploid budding yeast (Saccharomyces cerevisiae) can adopt one of several alternative differentiation fates in response to nutrient limitation, and each of these fates provides distinct biological functions. When different strain backgrounds are taken into account, these various fates occur in response to similar environmental cues, are regulated by the same signal transduction pathways, and share many of the same master regulators. I propose that the relationships between fate choice, environmental cues and signaling pathways are not Boolean, but involve graded levels of signals, pathway activation and master-regulator activity.