
Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.

Luminal acetylation of microtubules is not essential for Plasmodium berghei and Toxoplasma gondii survival
Acetylation of α-tubulin at lysine 40 is not essential for cytoskeletal stability in Plasmodium berghei or Toxoplasma gondii, suggesting redundancy and plasticity in microtubule regulation in these parasites.

The dual-site agonist for human M2 muscarinic receptors Iper-8-naphtalimide induces mitochondrial dysfunction in Saccharomyces cerevisiae
S. cerevisiae is a model to study human GPCRs. N-8-Iper, active against glioblastoma via M2 receptor, causes mitochondrial damage in yeast by binding Ste2, highlighting evolutionary conservation of GPCRs.

Integrative Omics reveals changes in the cellular landscape of peroxisome-deficient pex3 yeast cells
To uncover the consequences of peroxisome deficiency, we compared Saccharomyces cerevisiae wild-type with pex3 cells, which lack peroxisomes, employing quantitative proteomics and transcriptomics technologies.

Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
Rebekkah E. Pope1, Patrick Ballmann2, Lisa Whitworth3 and Rolf A. Prade1,*
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
Evelyn Tevere1,a, María G. Mediavilla1,a, Cecilia B. Di Capua1, Marcelo L. Merli1, Carlos Robello2,3, Luisa Berná2,4 and Julia A. Cricco
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.
Sir2 regulates selective autophagy in stationary-phase yeast cells
Ji-In Ryua, Juhye Junga, and Jeong-Yoon Kim
This study establishes Sir2 as a previously unrecognized regulator of selective autophagy during the stationary phase and highlight how cells dynamically control organelle degradation.

Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
Rebekkah E. Pope1, Patrick Ballmann2, Lisa Whitworth3 and Rolf A. Prade1,*
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
Evelyn Tevere1,a, María G. Mediavilla1,a, Cecilia B. Di Capua1, Marcelo L. Merli1, Carlos Robello2,3, Luisa Berná2,4 and Julia A. Cricco
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.
Sir2 regulates selective autophagy in stationary-phase yeast cells
Ji-In Ryua, Juhye Junga, and Jeong-Yoon Kim
This study establishes Sir2 as a previously unrecognized regulator of selective autophagy during the stationary phase and highlight how cells dynamically control organelle degradation.

Luminal acetylation of microtubules is not essential for Plasmodium berghei and Toxoplasma gondii survival
Thrishla Kumar1,a, Katharina Röver2,a, Johannes F. Stortz3,a, Annika M. Binder2,a, Benjamin Spreng2, Madlen Konert2, Markus Meissner1, Friedrich Frischknecht2,4 and Elena Jimenez-Ruiz1,*
Acetylation of α-tubulin at lysine 40 is not essential for cytoskeletal stability in Plasmodium berghei or Toxoplasma gondii, suggesting redundancy and plasticity in microtubule regulation in these parasites.

The dual-site agonist for human M2 muscarinic receptors Iper-8-naphtalimide induces mitochondrial dysfunction in Saccharomyces cerevisiae
Angela Cirigliano1,a, Antonia Amelina2,a, Elena Passarini2, Alessandra Ricelli1, Nicole Balasco1, Mattia Mori3, Bruno Botta4, Maria Egle De Stefano2,5, Claudio Papotto6, Claudia Guerriero2, Ada Maria Tata2,5 and Teresa Rinaldi2,*
S. cerevisiae is a model to study human GPCRs. N-8-Iper, active against glioblastoma via M2 receptor, causes mitochondrial damage in yeast by binding Ste2, highlighting evolutionary conservation of GPCRs.
Organelle activity organized by the endoplasmic reticulum-mitochondria encounter structure –ERMES– is essential for Podospora anserina development
Melisa Álvarez-Sánchez1, Matías Ramírez-Noguez1, Beatriz Aguirre-López1 and Leonardo Peraza-Reyes1
Eucaryotic cell functioning and development depend on the concerted activity of its organelles. In the model fungus Podospora anserina, sexual development involves a dynamic regulation of mitochondria, peroxisomes and the endoplasmic reticulum (ER), suggesting that their activity during this process is coordinated.
Role of the putative sit1 gene in normal germination of spores and virulence of the Mucor lusitanicus
Bernadett Vágó1,2, Kitti Bauer1,2, Naomi Varghese1,2, Sándor Kiss-Vetráb1,2, Sándor Kocsubé1,2, Mónika Varga1,2, András Szekeres1,2, Csaba Vágvölgyi1,2, Tamás Papp1,2,3,# and Gábor Nagy1,2,3,#
Mucormycosis is a life-threatening infection caused by certain members of the fungal order Mucorales, with increased incidence in recent years. Individuals with untreated diabetes mellitus, and patients treated with deferoxamine are particularly susceptible to this infection.
Tumor microenvironment signatures enhances lung adenocarcinoma prognosis prediction: Implication of intratumoral microbiota
Fei Zhao1,#, Lei Wang2,3,4,#, Dongjie Du5, Heaven Zhao6,7, Geng Tian6,7, Yufeng Li2,3,8, Yankun Liu2,8,9, Zhiwu Wang2,3,10, Dasheng Liu11, Jingwu Li2,3,12, Lei Ji6,7 and Hong Zhao1
The interaction between intratumoral microbiome and the tumor microenvironment (TME) has furthered our understanding of tumor ecology. Yet, the implications of their interaction for lung cancer management remain unclear.
From the Uncharacterized Protein Family 0016 to the GDT1 family: Molecular insights into a newly-characterized family of cation secondary transporters
Louise Thines1, Jiri Stribny1 and Pierre Morsomme1
This review outlines how the formerly uncharacterized UPF0016 family, now known as the Gdt1 family, plays key roles in cation transport – especially Mn²⁺ – across species from bacteria to humans. These proteins are crucial for processes like glycosylation, photosynthesis, and calcium signaling, with functions linked to their localization in membranes such as the Golgi, chloroplast, and plasma membrane and by that highlighting their evolutionary conservation and physiological relevance, offering insights into their shared and distinct features across organisms.
A broad-spectrum antibiotic adjuvant SLAP-S25: one stone many birds
Meirong Song1 and Kui Zhu1
This article refers to the study “A broad-spectrum antibiotic adjuvant reverses multidrug-resistant Gram-negative pathogens” by Song et al. (Nat Microbiol, 2020), which deals with the growing threat of antibiotic resistance, with few new drugs being developed for decades. The study found that the peptide SLAP-S25 enhances the efficacy of several antibiotics against resistant Gram-negative bacteria by disrupting their membranes, thereby increasing drug uptake. This suggests that bacterial membranes are promising targets for new antibiotic adjuvants.
Hiding in plain sight: vesicle-mediated export and transmission of prion-like proteins
Mehdi Kabani1
This article relates to the study “Glucose availability dictates the export of the soluble and prion forms of Sup35p via periplasmic or extracellular vesicles” by Kabani et al. (Mol Microbiol, 2020) that provides compelling evidence that yeast prions, such as Sup35p in its infectious [PSI⁺] state, can be exported via both extracellular vesicles (EVs) and periplasmic vesicles (PVs), with this export being modulated by environmental glucose levels. The discovery that prion particles are released in high amounts through PVs during glucose starvation adds a new dimension to our understanding of prion transmission and opens up fascinating possibilities for exploring vesicle-mediated spread of protein aggregates in neurodegenerative diseases using yeast as a model system.
Regulation of Cdc42 for polarized growth in budding yeast
Kristi E. Miller1,2, Pil Jung Kang1 and Hay-Oak Park1
This review highlights how studies in budding yeast have revealed a biphasic mechanism of Cdc42 activation that governs cell polarity establishment, with implications for understanding similar processes in mammalian cells and the role of Cdc42 in aging.
Yeast-based assays for the functional characterization of cancer-associated variants of human DNA repair genes
Tiziana Cervelli1, Samuele Lodovichi1, Francesca Bellè1 and Alvaro Galli1
This article highlights how the genetic tractability and conserved DNA repair pathways of yeast make it a powerful system for functionally characterizing human cancer-associated variants in DNA repair genes, aiding in risk assessment and therapeutic decision-making.
A novel c-di-GMP signal system regulates biofilm formation in Pseudomonas aeruginosa
Gukui Chen1 and Haihua Liang1
This article relates to the study “The SiaA/B/C/D signaling network regulates biofilm formation in Pseudomonas aeruginosa” by Chen et al. (EMBO J, 2020) that reveals a novel signaling network encoded by the siaABCD operon in Pseudomonas aeruginosa that regulates biofilm and aggregate formation by controlling the diguanylate cyclase activity of SiaD through phosphorylation-dependent interactions with SiaC, highlighting a potential antimicrobial target.
Regulation of anti-microbial autophagy by factors of the complement system
Christophe Viret1, Aurore Rozières1, Rémi Duclaux-Loras1, Gilles Boschetti1, Stéphane Nancey1 and
Mathias Faure1,2
This review explores emerging evidence that components of the complement system, beyond their traditional immune roles, modulate autophagy – particularly xenophagy – thereby influencing cell-autonomous antimicrobial responses during host-pathogen interactions.
More than flipping the lid: Cdc50 contributes to echinocandin resistance by regulating calcium homeostasis in Cryptococcus neoformans
Chengjun Cao1 and Chaoyang Xue1,2
In this article, the authors comment on the study “A mechanosensitive channel governs lipid flippase-mediated echinocandin resistance in Cryptococcus neoformans” by Cao et al. (mBio, 2019), which uncovers a dual role for the lipid flippase subunit Cdc50 in Cryptococcus neoformans, linking lipid translocation and calcium signaling via its interaction with the mechanosensitive channel Crm1, thereby contributing to innate resistance against the antifungal drug caspofungin.
The emerging role of complex modifications of tRNALysUUU in signaling pathways
Patrick C. Thiaville1,2,3,4 and Valérie de Crécy-Lagard2,4
This comment discusses the article “Loss of wobble uridine modification in tRNA anticodons interferes with TOR pathway signaling” by Scheidt et al (Microbial Cell, 2014).
Only functional localization is faithful localization
Roland Lill1,2,3
This article comments on work published by Peleh et al. (Microbial Cell 2014), which analyzes the localization of Dre2 in Saccharomyces cerevisiae.
One cell, one love: a journal for microbial research
Didac Carmona-Gutierrez1, Guido Kroemer2-6 and Frank Madeo1
In this inaugural article of Microbial Cell, we highlight the importance of microbial research in general and the journal’s intention to serve as a publishing forum that supports and enfolds the scientific diversity in this area as it provides a unique, high-quality and universally accessible source of information and inspiration.
What’s the role of autophagy in trypanosomes?
Katherine Figarella1 and Néstor L. Uzcátegui1,2
This article comments on Proto et al. (Microbial Cell, 2014), who report first insights into the molecular mechanism of autophagy in African trypanosomes by generating reporter bloodstream form cell lines.
Microbial Cell
is an open-access, peer-reviewed journal that publishes exceptionally relevant research works that implement the use of unicellular organisms (and multicellular microorganisms) to understand cellular responses to internal and external stimuli and/or human diseases.
you can trust
Can’t find what you’re looking for?
You can browse all our issues and published articles here.
FAQs
Peer-reviewed, open-access research using unicellular organisms (and multicellular microorganisms) to understand cellular responses and human disease.
The journal (founded in 2014) is led by its Editors-in-Chief Frank Madeo, Didac Carmona-Gutierrez, and Guido Kroemer
Microbial Cell has been publishing original scientific literature since 2014, and from the very beginning has been managed by active scientists through an independent Publishing House (Shared science Publishers). The journal was conceived as a platform to acknowledge the importance of unicellular organisms, both as model systems as well as in the biological context of human health and disease.
Ever since, Microbial Cell has very positively developed and strongly grown into a respected journal in the unicellular research community and even beyond. This scientific impact is reflected in the yearly number of citations obtained by articles published in Microbial Cell, as recorded by the Web of Science (Clarivate, formerly Thomson/Reuters):
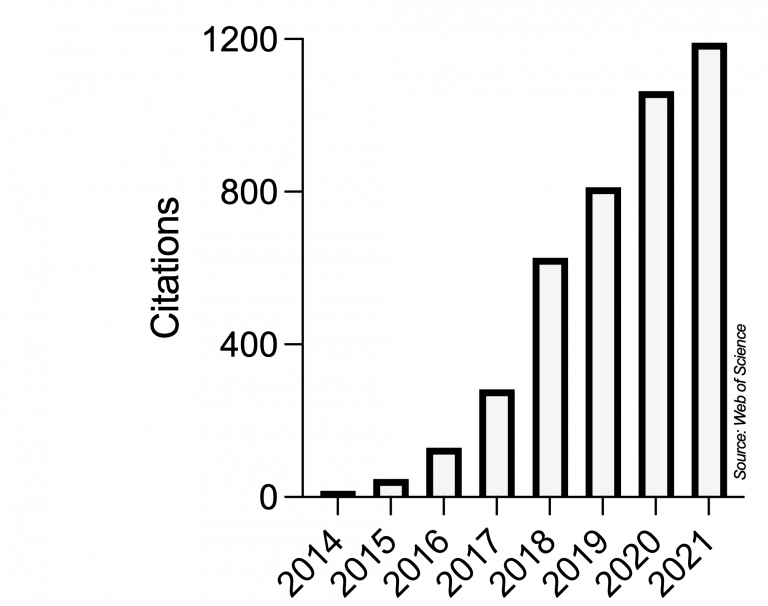
The scientific impact of Microbial Cell is also mirrored in a series of milestones:
2015: Microbial Cell is included in the Emerging Sources Citation Index (ESCI), a selection of developing journals drafted by Clarivate Analytics based on the candidate’s publishing standards, quality, editorial content, and citation data. Note: As an ESCI-selected journal, Microbial Cell is currently being evaluated in a rigorous and long process to determine an inclusion in the Science Citation Index Expanded (SCIE), which allows the official calculation of Clarivate Analytics’ impact factor.
2016: Microbial Cell is awarded the so-called DOAJ Seal by the selective Directory of Open Access Journals (DOAJ). The DOAJ Seal is an exclusive mark of certification for open access journals granted by DOAJ to journals that adhere to outstanding best practice and achieve an extra high and clear commitment to open access and high publishing standards.
2017: Microbial Cell is included in Pubmed Central (PMC), allowing the archiving of all the journal’s articles in PMC and PubMed.
2019: Microbial Cell is indexed in the prestigious abstract and citation database Scopus after a thorough selection process. This also means that Microbial Cell obtains, for the first time, an official Scopus CiteScore as well as an official journal ranking in the Scimago Journal and Country Ranking.
2022: Microbial Cell’s CiteScore reaches a value of 7.2 for the year 2021, positioning Microbial Cell among the top microbiology journals (previously available CiteScores: 2019: 5.4; 2020: 5.1).
2022: Microbial Cell is indexed in the highly selective Science Citation Index Expanded™, which covers approx. 9,500 of the world’s most impactful journals across 178 scientific disciplines. In their journal selection and curation process, Clarivate´s editors apply 24 ‘quality’ criteria and four ‘impact’ criteria to select the most influential journals in their respective fields. This selection is also a pre-requisite for inclusion in the JCR, which features the impact factor.
2022: Microbial Cell is listed in the Journal Citation Reports™ (JCR), and obtains its first official Journal Impact Factor™ (JIF) for the year 2021: 5.316.Check Article Types and Manuscript Preparation guidelines. Submit online via Scholastica.

Metabolic pathways further increase the complexity of cell size control in budding yeast
Jorrit M. Enserink
This article comments on work published by Soma et al. (Microbial Cell, 2014), which teased apart the effect of metabolism and growth rate on setting of critical cell size in Saccharomyces cerevisiae.