
Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.

Luminal acetylation of microtubules is not essential for Plasmodium berghei and Toxoplasma gondii survival
Acetylation of α-tubulin at lysine 40 is not essential for cytoskeletal stability in Plasmodium berghei or Toxoplasma gondii, suggesting redundancy and plasticity in microtubule regulation in these parasites.

The dual-site agonist for human M2 muscarinic receptors Iper-8-naphtalimide induces mitochondrial dysfunction in Saccharomyces cerevisiae
S. cerevisiae is a model to study human GPCRs. N-8-Iper, active against glioblastoma via M2 receptor, causes mitochondrial damage in yeast by binding Ste2, highlighting evolutionary conservation of GPCRs.

Integrative Omics reveals changes in the cellular landscape of peroxisome-deficient pex3 yeast cells
To uncover the consequences of peroxisome deficiency, we compared Saccharomyces cerevisiae wild-type with pex3 cells, which lack peroxisomes, employing quantitative proteomics and transcriptomics technologies.

Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
Rebekkah E. Pope1, Patrick Ballmann2, Lisa Whitworth3 and Rolf A. Prade1,*
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
Evelyn Tevere1,a, María G. Mediavilla1,a, Cecilia B. Di Capua1, Marcelo L. Merli1, Carlos Robello2,3, Luisa Berná2,4 and Julia A. Cricco
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.
Sir2 regulates selective autophagy in stationary-phase yeast cells
Ji-In Ryua, Juhye Junga, and Jeong-Yoon Kim
This study establishes Sir2 as a previously unrecognized regulator of selective autophagy during the stationary phase and highlight how cells dynamically control organelle degradation.
Stable and destabilized GFP reporters to monitor calcineurin activity in Saccharomyces cerevisiae
Jutta Diessl1, Arpita Nandy1, Christina Schug1, Lukas Habernig1 and Sabrina Büttner1,2
This study introduces GFP-based transcriptional reporters driven by a calcineurin-dependent response element, enabling real-time monitoring of calcineurin activity in live yeast cells for studying stress responses, aging, and antifungal drug screening.
The euchromatic histone mark H3K36me3 preserves heterochromatin through sequestration of an acetyltransferase complex in fission yeast
Paula R. Georgescu1, Matías Capella1, Sabine Fischer-Burkart1 and Sigurd Braun1
This study reveals that the loss of heterochromatin silencing in Set2-deficient cells is due to unrestrained Mst2C activity, highlighting the need for spatially restricted chromatin-modifying enzymes to maintain distinct chromatin states.
Depletion of SNAP-23 and Syntaxin 4 alters lipid droplet homeostasis during Chlamydia infection
Tiago Monteiro-Brás1,2,3, Jordan Wesolowski1 and Fabienne Paumet1
This study reveals that the plasma membrane SNARE proteins SNAP-23 and Syntaxin 4 are crucial for Chlamydia trachomatis development by regulating lipid droplet homeostasis and supporting the formation of infectious progeny within host cells.
Yeast can express and assemble bacterial secretins in the mitochondrial outer membrane
Janani Natarajan1, Anasuya Moitra1, Sussanne Zabel1,§, Nidhi Singh2, Samuel Wagner2,3 and Doron Rapaport1
Secretins, essential components of bacterial secretion systems, can be expressed in yeast and show differential dependencies on mitochondrial import and assembly factors for membrane integration, suggesting diverse pathways for their assembly into the bacterial outer membrane.
Metabolic reprogramming of Salmonella infected macrophages and its modulation by iron availability and the mTOR pathway
Julia Telser1,2,#, Chiara Volani1,3,#, Richard Hilbe1,2, Markus Seifert1,2, Natascha Brigo1, Giuseppe Paglia4 and Günter Weiss1,2
This article shows that iron plays a critical role in both the immune response and metabolic reprogramming of macrophages during infection, influencing the TCA cycle and mTOR pathway, with implications for the growth of intracellular bacteria like Salmonella.
Transcriptomic and chemogenomic analyses unveil the essential role of Com2-regulon in response and tolerance of Saccharomyces cerevisiae to stress induced by sulfur dioxide
Patrícia Lage1,2, Belém Sampaio-Marques3,4, Paula Ludovico3,4, Nuno P. Mira5 and Ana Mendes-Ferreira1,2
This article shows that in the presence of sulfur dioxide (SO2), the transcription factor Com2 plays a critical role in the tolerance and response of Saccharomyces cerevisiae, affecting the expression of a majority of SO2-activated genes and contributing to the protection against stress induced by SO2 at an enologically relevant pH.
Network dynamics of the yeast methyltransferome
Guri Giaever1, Elena Lissina1 and Corey Nislow1
This article presents a systematic genetic analysis of methyltransferases (MTases) under normal and stress conditions, uncovering the complex and adaptive nature of the methyltransferome and discovering a potential connection between phospholipid methylation and histone methylation, suggesting interplay between lipid homeostasis and epigenetic regulation.
New insights into the function of a versatile class of membrane molecular motors from studies of Myxococcus xanthus surface (gliding) motility
Tâm Mignot1 and Marcelo Nöllmann2
This article comments on work published by Faure et al. (Nature, 2016), which deciphers force transmission at focal adhesion complexes that are involved in gliding motility in bacteria.
Advancing host-directed therapy for tuberculosis: new therapeutic insights from the Toxoplasma gondii
Chul-Su Yang
This article comments on work published by Koh et al. (PLoS Pathog, 2017), which uncovered that infection-induced signaling pathways suggest possibilities for the development of novel therapeutic modalities for TB that target the intracellular signaling pathways permitting the replication of Mycobacterium tuberculosis (MTB).
Breaking the bilayer: OMV formation during environmental transitions
Katherine E. Bonnington, Meta J. Kuehn
This article comments on work published by Bonnington & Kuehn (MBio, 2016), which shows how gram-negative bacteria maintain the barrier properties of the outer membrane (OM) in a wide array of physiological conditions despite their inability to degrade lipopolysaccharide (LPS) and protein material present in the outer leaflet of the OM.
The tug-of-war over MTOR in Legionella infections
Stanimir S. Ivanov
This article comments on work published by Abshire et al (PLoS Pathog, 2016), which uncovered that the host metabolic checkpoint kinase Mechanistic target of rapamycin (MTOR) is a central regulator of the pathogen niche expansion program.
A new role for Holliday junction resolvase Yen1 in processing DNA replication intermediates exposes Dna2 as an accessory replicative helicase
Benoît Falquet1,2 and Ulrich Rass
This article comments on work published by Ölmezer et al. (Nat Commun, 2016), which revealed a new function of Yen1, distinct from its previously known role as a Holliday junction resolvase, mediating the removal of branched HR intermediates.
Toxin-mediated gene regulatory mechanism in Staphylococcus aureus
Hwang-Soo Joo and Michael Otto
This article comments on work published by Joo et al. (MBio, 2016), which describes the first molecular regulatory mechanism exerted by an S. aureus toxin, setting a paradigmatic example of how S. aureus toxins may influence cell functions to adjust them to times of toxin production.
Autophagy: machinery and regulation
Zhangyuan Yin, Clarence Pascual and Daniel J. Klionsky
Macroautophagy/autophagy is an evolutionarily conserved cellular degradation process that targets cytoplasmic materials including cytosol, macromolecules and unwanted organelles. The discovery and analysis of autophagy-related (Atg) proteins have unveiled much of the machinery of autophagosome formation. In this review, we briefly summarize the physiological roles, molecular mechanism, regulatory network, and pathophysiological roles of autophagy.
NprR, a moonlighting quorum sensor shifting from a phosphatase activity to a transcriptional activator
Stéphane Perchat1, Antoine Talagas2, Samira Zouhir2, Sandrine Poncet1, Laurent Bouillaut1,¶, Sylvie Nessler2 and Didier Lereclus1
This article comments on work published by Perchat et al. (PLoS Pathog, 2016), which demonstrates that, in the absence of the signaling peptide NprX, the sensor NprR is a dimer, which negatively controls sporulation in Bacillus thuringiensis, independently of its transcription factor activity.
Threading Granules in Freiburg: 2nd International Symposium on “One Mitochondrion, Many Diseases – Biological and Molecular Perspectives”, a FRIAS Junior Researcher Conference, Freiburg im Breisgau, Germany, March 9th/10th, 2016
Ralf J. Braun1, Ralf M. Zerbes2, Florian Steinberg3, Denis Gris4, and Verónica I. Dumit5
INTRODUCTION Mitochondria (greek: μίτος & χονδρίον, mitos & chondrion, i.e., thread & granule) are the power houses of eukaryotic cells, and are pivotally involved in essential metabolic processes, including iron/sulfur cluster and heme biosynthesis. Mitochondria
The emerging role of complex modifications of tRNALysUUU in signaling pathways
Patrick C. Thiaville1,2,3,4 and Valérie de Crécy-Lagard2,4
This comment discusses the article “Loss of wobble uridine modification in tRNA anticodons interferes with TOR pathway signaling” by Scheidt et al (Microbial Cell, 2014).
Only functional localization is faithful localization
Roland Lill1,2,3
This article comments on work published by Peleh et al. (Microbial Cell 2014), which analyzes the localization of Dre2 in Saccharomyces cerevisiae.
One cell, one love: a journal for microbial research
Didac Carmona-Gutierrez1, Guido Kroemer2-6 and Frank Madeo1
In this inaugural article of Microbial Cell, we highlight the importance of microbial research in general and the journal’s intention to serve as a publishing forum that supports and enfolds the scientific diversity in this area as it provides a unique, high-quality and universally accessible source of information and inspiration.
What’s the role of autophagy in trypanosomes?
Katherine Figarella1 and Néstor L. Uzcátegui1,2
This article comments on Proto et al. (Microbial Cell, 2014), who report first insights into the molecular mechanism of autophagy in African trypanosomes by generating reporter bloodstream form cell lines.
Microbial Cell
is an open-access, peer-reviewed journal that publishes exceptionally relevant research works that implement the use of unicellular organisms (and multicellular microorganisms) to understand cellular responses to internal and external stimuli and/or human diseases.
you can trust
Can’t find what you’re looking for?
You can browse all our issues and published articles here.
FAQs
Peer-reviewed, open-access research using unicellular organisms (and multicellular microorganisms) to understand cellular responses and human disease.
The journal (founded in 2014) is led by its Editors-in-Chief Frank Madeo, Didac Carmona-Gutierrez, and Guido Kroemer
Microbial Cell has been publishing original scientific literature since 2014, and from the very beginning has been managed by active scientists through an independent Publishing House (Shared science Publishers). The journal was conceived as a platform to acknowledge the importance of unicellular organisms, both as model systems as well as in the biological context of human health and disease.
Ever since, Microbial Cell has very positively developed and strongly grown into a respected journal in the unicellular research community and even beyond. This scientific impact is reflected in the yearly number of citations obtained by articles published in Microbial Cell, as recorded by the Web of Science (Clarivate, formerly Thomson/Reuters):
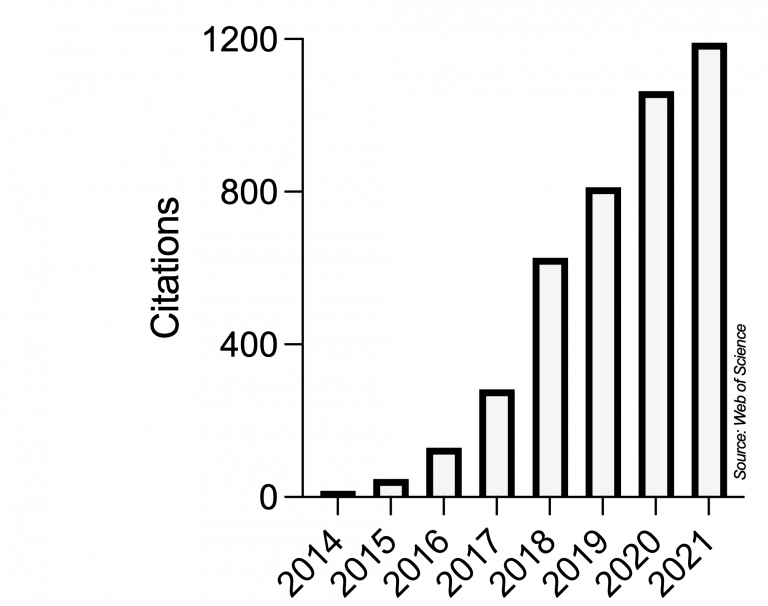
The scientific impact of Microbial Cell is also mirrored in a series of milestones:
2015: Microbial Cell is included in the Emerging Sources Citation Index (ESCI), a selection of developing journals drafted by Clarivate Analytics based on the candidate’s publishing standards, quality, editorial content, and citation data. Note: As an ESCI-selected journal, Microbial Cell is currently being evaluated in a rigorous and long process to determine an inclusion in the Science Citation Index Expanded (SCIE), which allows the official calculation of Clarivate Analytics’ impact factor.
2016: Microbial Cell is awarded the so-called DOAJ Seal by the selective Directory of Open Access Journals (DOAJ). The DOAJ Seal is an exclusive mark of certification for open access journals granted by DOAJ to journals that adhere to outstanding best practice and achieve an extra high and clear commitment to open access and high publishing standards.
2017: Microbial Cell is included in Pubmed Central (PMC), allowing the archiving of all the journal’s articles in PMC and PubMed.
2019: Microbial Cell is indexed in the prestigious abstract and citation database Scopus after a thorough selection process. This also means that Microbial Cell obtains, for the first time, an official Scopus CiteScore as well as an official journal ranking in the Scimago Journal and Country Ranking.
2022: Microbial Cell’s CiteScore reaches a value of 7.2 for the year 2021, positioning Microbial Cell among the top microbiology journals (previously available CiteScores: 2019: 5.4; 2020: 5.1).
2022: Microbial Cell is indexed in the highly selective Science Citation Index Expanded™, which covers approx. 9,500 of the world’s most impactful journals across 178 scientific disciplines. In their journal selection and curation process, Clarivate´s editors apply 24 ‘quality’ criteria and four ‘impact’ criteria to select the most influential journals in their respective fields. This selection is also a pre-requisite for inclusion in the JCR, which features the impact factor.
2022: Microbial Cell is listed in the Journal Citation Reports™ (JCR), and obtains its first official Journal Impact Factor™ (JIF) for the year 2021: 5.316.Check Article Types and Manuscript Preparation guidelines. Submit online via Scholastica.

Metabolic pathways further increase the complexity of cell size control in budding yeast
Jorrit M. Enserink
This article comments on work published by Soma et al. (Microbial Cell, 2014), which teased apart the effect of metabolism and growth rate on setting of critical cell size in Saccharomyces cerevisiae.