
Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.

Luminal acetylation of microtubules is not essential for Plasmodium berghei and Toxoplasma gondii survival
Acetylation of α-tubulin at lysine 40 is not essential for cytoskeletal stability in Plasmodium berghei or Toxoplasma gondii, suggesting redundancy and plasticity in microtubule regulation in these parasites.

The dual-site agonist for human M2 muscarinic receptors Iper-8-naphtalimide induces mitochondrial dysfunction in Saccharomyces cerevisiae
S. cerevisiae is a model to study human GPCRs. N-8-Iper, active against glioblastoma via M2 receptor, causes mitochondrial damage in yeast by binding Ste2, highlighting evolutionary conservation of GPCRs.

Integrative Omics reveals changes in the cellular landscape of peroxisome-deficient pex3 yeast cells
To uncover the consequences of peroxisome deficiency, we compared Saccharomyces cerevisiae wild-type with pex3 cells, which lack peroxisomes, employing quantitative proteomics and transcriptomics technologies.

Regulation of extracellular vesicles for protein secretion in Aspergillus nidulans
Rebekkah E. Pope1, Patrick Ballmann2, Lisa Whitworth3 and Rolf A. Prade1,*
This study reveals that Aspergillus nidulans boosts extracellular vesicle production when ER-trafficked enzymes are induced, uncovering how fungi remodel their secretome through vesicle-mediated secretion to adapt to changing environments and biofilm formation.

Transcriptomic response to different heme sources in Trypanosoma cruzi epimastigotes
Evelyn Tevere1,a, María G. Mediavilla1,a, Cecilia B. Di Capua1, Marcelo L. Merli1, Carlos Robello2,3, Luisa Berná2,4 and Julia A. Cricco
This study uncovers how the Chagas disease parasite adapts to changes in heme, an essential molecule for its survival, providing transcriptional clues to heme metabolism and identifying a previously unreported heme-binding protein in T. cruzi.
Sir2 regulates selective autophagy in stationary-phase yeast cells
Ji-In Ryua, Juhye Junga, and Jeong-Yoon Kim
This study establishes Sir2 as a previously unrecognized regulator of selective autophagy during the stationary phase and highlight how cells dynamically control organelle degradation.
Global translational impacts of the loss of the tRNA modification t6A in yeast
Patrick C. Thiaville1,2,3,4, Rachel Legendre4, Diego Rojas-Benítez5, Agnès Baudin-Baillieu4, Isabelle Hatin4, Guilhem Chalancon6, Alvaro Glavic5, Olivier Namy4, Valérie de Crécy-Lagard1,3
The universal tRNA modification t6A is found at position 37 of nearly all tRNAs decoding ANN codons. Analysis of codon occupancy rates suggests that one of the major roles of t6A is to homogenize the process of elongation by slowing the elongation rate at codons decoded by high abundance tRNAs and I34:C3 pairs while increasing the elongation rate of rare tRNAs and G34:U3 pairs. This work reveals that the consequences of t6A absence are complex and multilayered and has set the stage to elucidate the molecular basis of the observed phenotypes.
Ergosterone-coupled Triazol molecules trigger mitochondrial dysfunction, oxidative stress, and acidocalcisomal Ca2+ release in Leishmania mexicana promastigotes
Figarella K1, Marsiccobetre S1, Arocha I1, Colina W2, Hasegawa M2,†, Rodriguez M2, Rodriguez-Acosta A3, Duszenko M4, Benaim G5, Uzcategui NL3
The protozoan parasite Leishmania causes a variety of sicknesses with different clinical manifestations known as leishmaniasis. Investigations looking for new targets or new active molecules focus mainly on the disruption of parasite specific pathways. In this sense, ergosterol biosynthesis is one of the most attractive because it does not occur in mammals. Our results indicate that ergosterone-triazol coupled molecules induce a regulated cell death process in the parasite and may represent starting point molecules in the search of new chemotherapeutic agents to combat leishmaniasis.
INO1 transcriptional memory leads to DNA zip code-dependent interchromosomal clustering
Donna Garvey Brickner, Robert Coukos and Jason H. Brickner
Many genes localize at the nuclear periphery through physical interaction with the nuclear pore complex (NPC). We have found that the yeast INO1 gene is targeted to the NPC both upon activation and for several generations after repression, a phenomenon called epigenetic transcriptional memory. Targeting of INO1 to the NPC requires distinct cis-acting promoter DNA zip codes under activating conditions and under memory conditions. When at the nuclear periphery, active INO1 clusters with itself and with other genes that share the GRS I zip code. Here, we show that during memory, the two alleles of INO1 cluster in diploids and endogenous INO1 clusters with an ectopic INO1 in haploids. After repression, INO1 does not cluster with GRS I – containing genes. Furthermore, clustering during memory requires Nup100 and two sets of DNA zip codes…
A central role for TOR signalling in a yeast model for juvenile CLN3 disease
Michael E. Bond1, Rachel Brown1, Charalampos Rallis3,4, Jürg Bähler3,4 and Sara E. Mole1,2,3
Yeasts provide an excellent genetically tractable eukaryotic system for investigating the function of genes in their biological context, and are especially relevant for those conserved genes that cause disease. Bond et al. study the role of btn1, the orthologue of a human gene that underlies an early onset neurodegenerative disease (juvenile CLN3 disease, neuronal ceroid lipofuscinosis (NCLs) or Batten disease) in the fission yeast Schizosaccharomyces pombe.
Oxygen availability strongly affects chronological lifespan and thermotolerance in batch cultures of Saccharomyces cerevisiae
Markus M.M. Bisschops1,3,#, Tim Vos1,#, Rubén Martínez-Moreno2,4, Pilar de la Torre Cortés1, Jack T. Pronk1, Pascale Daran-Lapujade1
Stationary-phase (SP) batch cultures of Saccharomyces cerevisiae, in which growth has been arrested by carbon-source depletion, are widely applied to study chronological lifespan, quiescence and SP-associated robustness. Based on this type of experiments, typically performed under aerobic conditions, several roles of oxygen in aging have been proposed. However, SP in anaerobic yeast cultures has not been investigated in detail. Here, we use the unique capability of S. cerevisiae to grow in the complete absence of oxygen to directly compare SP in aerobic and anaerobic bioreactor cultures. This comparison revealed strong positive effects of oxygen availability on adenylate energy charge, longevity and thermotolerance during SP. A low thermotolerance of…
DNA damage checkpoint adaptation genes are required for division of cells harbouring eroded telomeres
Sofiane Y. Mersaoui, Serge Gravel, Victor Karpov, and Raymund J. Wellinger
In budding yeast, telomerase and the Cdc13p protein are two key players acting to ensure telomere stability. This article shows that while the capping process can be flexible, it takes a very specific genetic setup to allow a change from canonical capping to alternative capping.
The MAPKKKs Ste11 and Bck1 jointly transduce the high oxidative stress signal through the cell wall integrity MAP kinase pathway
Chunyan Jin#, Stephen K. Kim, Stephen D. Willis and Katrina F. Cooper
Oxidative stress stimulates the Rho1 GTPase, which in turn induces the cell wall integrity (CWI) MAP kinase cascade. CWI activation promotes stress-responsive gene expression through activation of transcription factors (Rlm1, SBF) and nuclear release and subsequent destruction of the repressor cyclin C. This study reports that, in response to high hydrogen peroxide exposure, or in the presence of constitutively active Rho1, cyclin C still translocates to the cytoplasm and is degraded in cells lacking Bck1, the MAPKKK of the CWI pathway.
Formyl-methionine as a degradation signal at the N-termini of bacterial proteins
Konstantin I. Piatkov1,3,#, Tri T. M. Vu1,#, Cheol-Sang Hwang2 and Alexander Varshavsky1
Varshavsky and colleagues solve a long-standing mystery in proteolysis! In bacteria, all nascent proteins bear the pretranslationally formed N-terminal formyl-methionine (fMet) residue. The fMet residue is cotranslationally deformylated by a ribosome-associated deformylase. The formylation of N-terminal Met in bacterial proteins is not strictly essential for either translation or cell viability. Moreover, protein synthesis by the cytosolic ribosomes of eukaryotes does not involve the formylation of N-terminal Met. What, then, is the main biological function of this metabolically costly, transient, and not strictly essential modification of N‑terminal Met, and why has Met formylation not been eliminated during bacterial evolution? One possibility is that the similarity of the formyl and acetyl groups, their identical locations in…
A Cinderella story: how the vacuolar proteases Pep4 and Prb1 do more than cleaning up the cell’s mass degradation processes
Winnie Kerstens1,2 and Patrick Van Dijck1,2
This review summarizes the expanded roles of the Saccharomyces cerevisiae vacuolar proteases Pep4 and Prb1 in non-vacuolar activities outside of autophagy, such as programmed cell death, protection from harmful protein forms, and gene expression regulation. The potential implications of these findings for fungal biology and drug target discovery, including insights for mammalian cell studies, are highlighted, emphasizing the need for a deeper understanding of these molecular processes.
The biosynthesis of pyoverdines
Michael T. Ringel1 and Thomas Brüser1
This review provides an overview of pyoverdine biosynthesis, emphasizing the distinctive fluorophore shared by various pyoverdines derived from ferribactins and the role of periplasmic processes in the maturation and modification of these siderophores, critical for the growth and colonization of hosts by fluorescent pseudomonads.
Toxin release mediated by the novel autolysin Cwp19 in Clostridium difficile
Imane El Meouche1 and Johann Peltier2,3
In this article, the authors comment on the study “Cwp19 is a novel lytic transglycosylase involved in stationary-phase autolysis resulting in toxin release in Clostridium difficile” by Wydau-Dematteis (MBio, 2018) that characterizes a novel peptidoglycan hydrolase, Cwp19, in Clostridioides difficile, highlighting its glucose-dependent mediation of toxins secretion and suggesting a potential role in the pathogenesis of this bacterium, contributing to the understanding of these enzymes in C. difficile and their implication in pathogenicity.
A global view of substrate phosphorylation and dephosphorylation during budding yeast mitotic exit
Sandra A. Touati1 and Frank Uhlmann1
In this article, the authors comment on the study “Phosphoproteome dynamics during mitotic exit in budding yeast” by Touati (EMBO J, 2018) that described a time-resolved global phosphoproteome analysis during a cell cycle phase known as mitotic exit in budding yeast revealed the principles of phosphoregulation governing the ordered sequence of events such as spindle elongation, chromosome decondensation, and completion of cell division.
Gammaretroviruses tether to mitotic chromatin by directly binding nucleosomal histone proteins
Madushi Wanaguru1 and Kate N. Bishop1
In this article, the authors comment on the study “Murine leukemia virus p12 tethers the capsid-containing pre-integration complex to chromatin by binding directly to host nucleosomes in mitosis” by Wanaguruet al. (PLoS Pathog, 2018) that highlights the essential role of the gammaretroviral gag cleavage product, p12, at both early and late stages of the virus life cycle, particularly in the integration of the viral DNA into the host cell chromatin to form a provirus. It also emphasizes the recent findings regarding the N- and C-terminal domains of p12, revealing their direct binding to the viral capsid lattice and nucleosomal histone proteins, respectively, thus elucidating the mechanism by which p12 links the viral pre-integration complex to mitotic chromatin.
Methodologies for in vitro and in vivo evaluation of efficacy of antifungal and antibiofilm agents and surface coatings against fungal biofilms
Patrick Van Dijck1,2,‡, Jelmer Sjollema3,‡, Bruno P.A. Cammue4,5, Katrien Lagrou6,7, Judith Berman8, Christophe d’Enfert9, David R. Andes10,11, Maiken C. Arendrup12-14, Axel A. Brakhage15, Richard Calderone16, Emilia Cantón17, Tom Coenye18,19, Paul Cos20, Leah E. Cowen21, Mira Edgerton22, Ana Espinel-Ingroff23, Scott G. Filler24, Mahmoud Ghannoum25, Neil A.R. Gow26, Hubertus Haas27, Mary Ann Jabra-Rizk28, Elizabeth M. Johnson29, Shawn R. Lockhart30, Jose L. Lopez-Ribot31, Johan Maertens32, Carol A. Munro26, Jeniel E. Nett33, Clarissa J. Nobile34, Michael A. Pfaller35,36, Gordon Ramage19,37, Dominique Sanglard38, Maurizio Sanguinetti39, Isabel Spriet40, Paul E. Verweij41, Adilia Warris42, Joost Wauters43, Michael R. Yeaman44, Sebastian A.J. Zaat45, Karin Thevissen4,*
This article highlights the critical importance of accurate susceptibility testing methods and the discovery of novel antifungal and antibiofilm agents in combating invasive fungal infections associated with biofilm formation on medical devices, thereby emphasizing the need for advancements in medical mycology research to address these complex diseases.
Shepherding DNA ends: Rif1 protects telomeres and chromosome breaks
Gabriele A. Fontana1, Julia K. Reinert1,2, Nicolas H. Thomä1, Ulrich Rass1
This review discusses the conserved mechanisms cells have evolved to protect DNA ends at chromosomal termini and DNA double-strand breaks (DSBs), focusing on the protein Rif1’s roles in telomere homeostasis and DSB repair in eukaryotes. It highlights the intriguing connection between Rif1’s involvement in both telomere maintenance and DSB repair, and suggests that excluding end-processing factors may underlie Rif1’s diverse biological functions at telomeres and chromosome breaks.
The CRISPR conundrum: evolve and maybe die, or survive and risk stagnation
Jesús García-Martínez1, Rafael D. Maldonado1, Noemí M. Guzmán1 and Francisco J. M. Mojica1,2
In this article García-Martínez et al. cover how the model bacterium Escherichia coli deals with CRISPR-Cas to tackle the major dilemma of evolution versus survival.
The emerging role of complex modifications of tRNALysUUU in signaling pathways
Patrick C. Thiaville1,2,3,4 and Valérie de Crécy-Lagard2,4
This comment discusses the article “Loss of wobble uridine modification in tRNA anticodons interferes with TOR pathway signaling” by Scheidt et al (Microbial Cell, 2014).
Only functional localization is faithful localization
Roland Lill1,2,3
This article comments on work published by Peleh et al. (Microbial Cell 2014), which analyzes the localization of Dre2 in Saccharomyces cerevisiae.
One cell, one love: a journal for microbial research
Didac Carmona-Gutierrez1, Guido Kroemer2-6 and Frank Madeo1
In this inaugural article of Microbial Cell, we highlight the importance of microbial research in general and the journal’s intention to serve as a publishing forum that supports and enfolds the scientific diversity in this area as it provides a unique, high-quality and universally accessible source of information and inspiration.
What’s the role of autophagy in trypanosomes?
Katherine Figarella1 and Néstor L. Uzcátegui1,2
This article comments on Proto et al. (Microbial Cell, 2014), who report first insights into the molecular mechanism of autophagy in African trypanosomes by generating reporter bloodstream form cell lines.
Microbial Cell
is an open-access, peer-reviewed journal that publishes exceptionally relevant research works that implement the use of unicellular organisms (and multicellular microorganisms) to understand cellular responses to internal and external stimuli and/or human diseases.
you can trust
Can’t find what you’re looking for?
You can browse all our issues and published articles here.
FAQs
Peer-reviewed, open-access research using unicellular organisms (and multicellular microorganisms) to understand cellular responses and human disease.
The journal (founded in 2014) is led by its Editors-in-Chief Frank Madeo, Didac Carmona-Gutierrez, and Guido Kroemer
Microbial Cell has been publishing original scientific literature since 2014, and from the very beginning has been managed by active scientists through an independent Publishing House (Shared science Publishers). The journal was conceived as a platform to acknowledge the importance of unicellular organisms, both as model systems as well as in the biological context of human health and disease.
Ever since, Microbial Cell has very positively developed and strongly grown into a respected journal in the unicellular research community and even beyond. This scientific impact is reflected in the yearly number of citations obtained by articles published in Microbial Cell, as recorded by the Web of Science (Clarivate, formerly Thomson/Reuters):
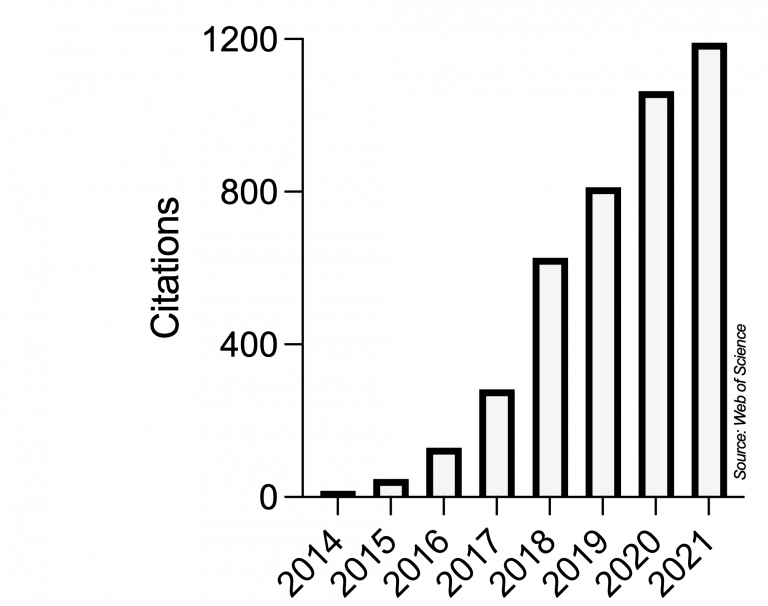
The scientific impact of Microbial Cell is also mirrored in a series of milestones:
2015: Microbial Cell is included in the Emerging Sources Citation Index (ESCI), a selection of developing journals drafted by Clarivate Analytics based on the candidate’s publishing standards, quality, editorial content, and citation data. Note: As an ESCI-selected journal, Microbial Cell is currently being evaluated in a rigorous and long process to determine an inclusion in the Science Citation Index Expanded (SCIE), which allows the official calculation of Clarivate Analytics’ impact factor.
2016: Microbial Cell is awarded the so-called DOAJ Seal by the selective Directory of Open Access Journals (DOAJ). The DOAJ Seal is an exclusive mark of certification for open access journals granted by DOAJ to journals that adhere to outstanding best practice and achieve an extra high and clear commitment to open access and high publishing standards.
2017: Microbial Cell is included in Pubmed Central (PMC), allowing the archiving of all the journal’s articles in PMC and PubMed.
2019: Microbial Cell is indexed in the prestigious abstract and citation database Scopus after a thorough selection process. This also means that Microbial Cell obtains, for the first time, an official Scopus CiteScore as well as an official journal ranking in the Scimago Journal and Country Ranking.
2022: Microbial Cell’s CiteScore reaches a value of 7.2 for the year 2021, positioning Microbial Cell among the top microbiology journals (previously available CiteScores: 2019: 5.4; 2020: 5.1).
2022: Microbial Cell is indexed in the highly selective Science Citation Index Expanded™, which covers approx. 9,500 of the world’s most impactful journals across 178 scientific disciplines. In their journal selection and curation process, Clarivate´s editors apply 24 ‘quality’ criteria and four ‘impact’ criteria to select the most influential journals in their respective fields. This selection is also a pre-requisite for inclusion in the JCR, which features the impact factor.
2022: Microbial Cell is listed in the Journal Citation Reports™ (JCR), and obtains its first official Journal Impact Factor™ (JIF) for the year 2021: 5.316.Check Article Types and Manuscript Preparation guidelines. Submit online via Scholastica.

Metabolic pathways further increase the complexity of cell size control in budding yeast
Jorrit M. Enserink
This article comments on work published by Soma et al. (Microbial Cell, 2014), which teased apart the effect of metabolism and growth rate on setting of critical cell size in Saccharomyces cerevisiae.